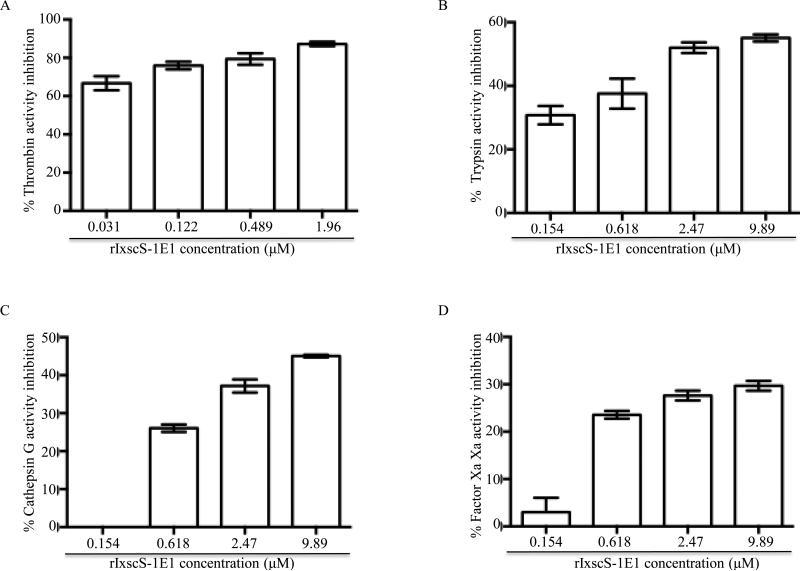
Fig. 3.
Inhibitor function profiling of recombinant Ixodes scapularis serpin (rIxscS-1E1). Various amounts of affinity purified rIxscS-1E1 (indicated) were pre-incubated with (A) 0.54 μM thrombin, (B) 6.68 μM trypsin, (C) 0.267 μ,M cathepsin G, (D) 0.0217 μM factor Xafor 10min at 37°C. Following incubation, peptide substrates were added and hydrolysis continuously monitored every 20 s up to 30min at A405nm using the VersaMax microplate reader. Substrate hydrolysis data was acquired using the SoftMax Pro software set to the default kinetics parameters to estimate end point maximum enzyme velocity (Vmax) or residual enzyme activity (REA). Vmax or REA at different rIxscS-1E1 concentrations were expressed as percentages of Vmax in the absence of rIxscS-1E1. Subtracting the percentage of REA from the assumed 100% enzyme activity in the absence of rIxscS-1E1 calculated the percentage of enzyme inhibition levels summarized as the mean ± S.E.M. of two assays.