Abstract
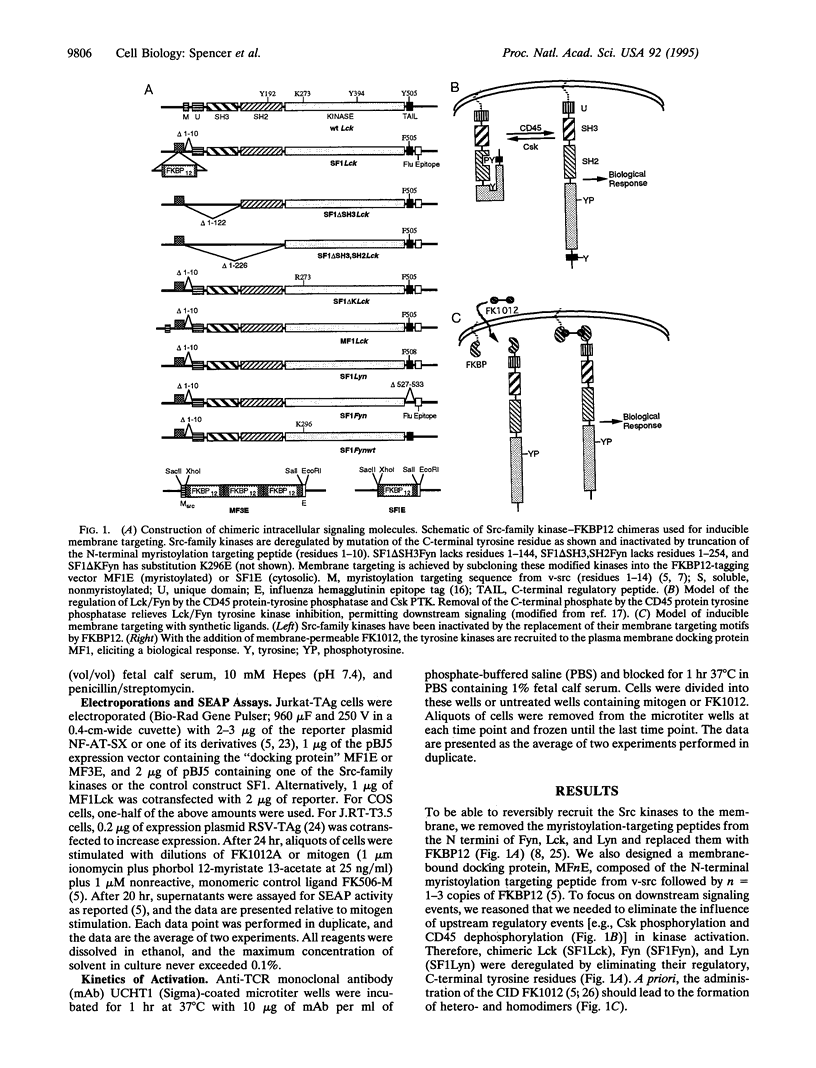
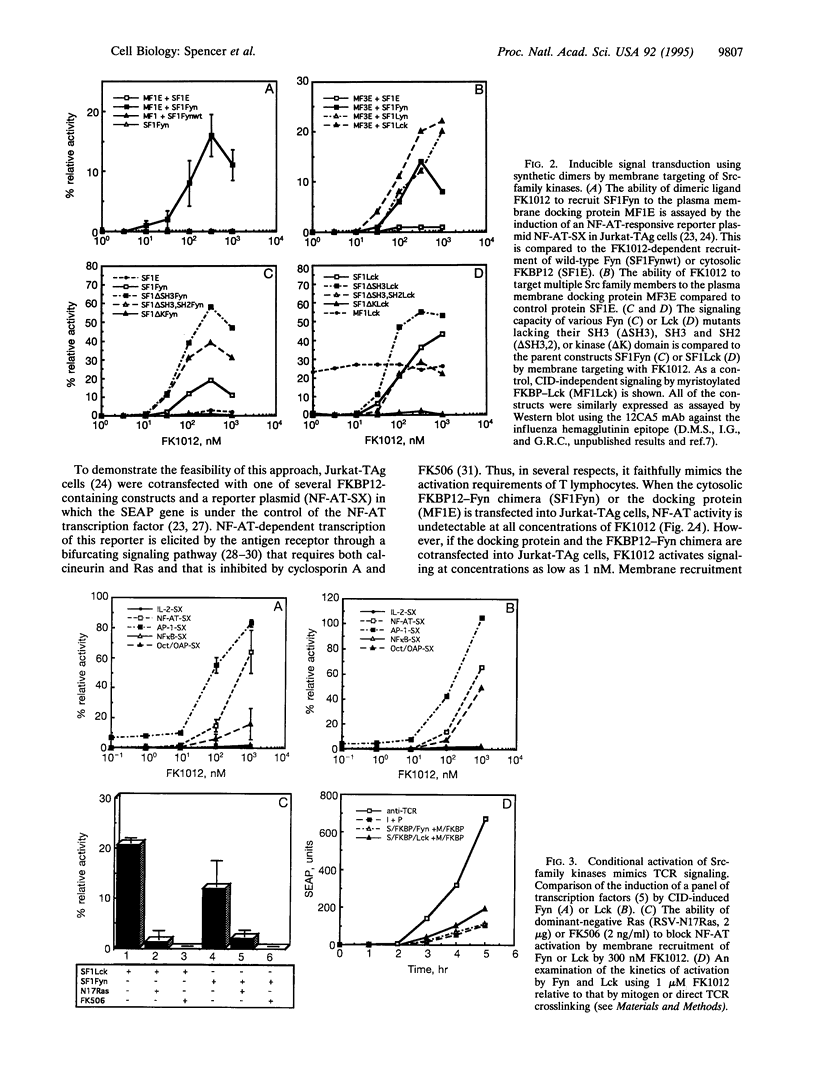
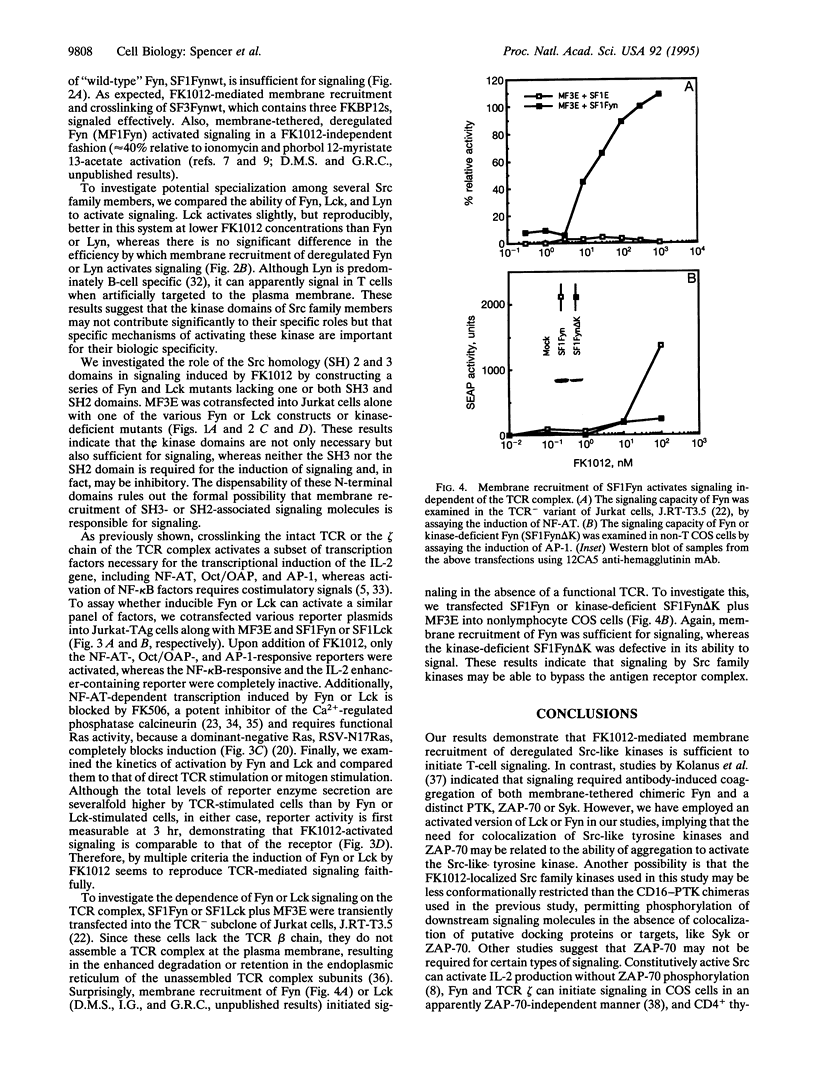
The Src-like tyrosine kinases require membrane localization for transformation and probably for their normal role in signal transduction. We utilized this characteristic to prepare Src-like tyrosine kinases that can be readily activated with the rationally designed chemical inducer of dimerization FK1012. Dimerization of cytoplasmic Src-like tyrosine kinases was not sufficient for signaling, but their recruitment to the plasma membrane led to the rapid activation of transcription factors identical to those regulated by crosslinking the antigen receptor. Moreover, recruitment of activated Src-like kinases to the membrane replaced signaling by the T-lymphocyte antigen receptor complex, leading to the activation of both the Ras/protein kinase C and Ca2+/calcineurin pathways normally activated by antigen receptor signaling. Since these chemical inducers of dimerization are cell permeable, this approach permits the production of conditional alleles of any of the Src-like tyrosine kinases, thereby allowing a delineation of their developmental roles.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alber T. Mutational effects on protein stability. Annu Rev Biochem. 1989;58:765–798. doi: 10.1146/annurev.bi.58.070189.004001. [DOI] [PubMed] [Google Scholar]
- Amrein K. E., Sefton B. M. Mutation of a site of tyrosine phosphorylation in the lymphocyte-specific tyrosine protein kinase, p56lck, reveals its oncogenic potential in fibroblasts. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4247–4251. doi: 10.1073/pnas.85.12.4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby M. W., Gross J. A., Cooke M. P., Levin S. D., Qian X., Perlmutter R. M. Defective T cell receptor signaling in mice lacking the thymic isoform of p59fyn. Cell. 1992 Sep 4;70(5):751–763. doi: 10.1016/0092-8674(92)90309-z. [DOI] [PubMed] [Google Scholar]
- Arpaia E., Shahar M., Dadi H., Cohen A., Roifman C. M. Defective T cell receptor signaling and CD8+ thymic selection in humans lacking zap-70 kinase. Cell. 1994 Mar 11;76(5):947–958. doi: 10.1016/0092-8674(94)90368-9. [DOI] [PubMed] [Google Scholar]
- Bergman M., Mustelin T., Oetken C., Partanen J., Flint N. A., Amrein K. E., Autero M., Burn P., Alitalo K. The human p50csk tyrosine kinase phosphorylates p56lck at Tyr-505 and down regulates its catalytic activity. EMBO J. 1992 Aug;11(8):2919–2924. doi: 10.1002/j.1460-2075.1992.tb05361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Suzuki C. K., Lippincott-Schwartz J., Weissman A. M., Klausner R. D. Pre-Golgi degradation of newly synthesized T-cell antigen receptor chains: intrinsic sensitivity and the role of subunit assembly. J Cell Biol. 1989 Jul;109(1):73–83. doi: 10.1083/jcb.109.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A. C., Kadlecek T. A., Elder M. E., Filipovich A. H., Kuo W. L., Iwashima M., Parslow T. G., Weiss A. ZAP-70 deficiency in an autosomal recessive form of severe combined immunodeficiency. Science. 1994 Jun 10;264(5165):1599–1601. doi: 10.1126/science.8202713. [DOI] [PubMed] [Google Scholar]
- Chen C., Bonifacino J. S., Yuan L. C., Klausner R. D. Selective degradation of T cell antigen receptor chains retained in a pre-Golgi compartment. J Cell Biol. 1988 Dec;107(6 Pt 1):2149–2161. doi: 10.1083/jcb.107.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. M., Fournel M., Davidson D., Veillette A. Negative regulation of T-cell receptor signalling by tyrosine protein kinase p50csk. Nature. 1993 Sep 9;365(6442):156–160. doi: 10.1038/365156a0. [DOI] [PubMed] [Google Scholar]
- Clipstone N. A., Crabtree G. R. Identification of calcineurin as a key signalling enzyme in T-lymphocyte activation. Nature. 1992 Jun 25;357(6380):695–697. doi: 10.1038/357695a0. [DOI] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Pellman D., Hanafusa H. A short sequence in the p60src N terminus is required for p60src myristylation and membrane association and for cell transformation. Mol Cell Biol. 1984 Sep;4(9):1834–1842. doi: 10.1128/mcb.4.9.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eck M. J., Atwell S. K., Shoelson S. E., Harrison S. C. Structure of the regulatory domains of the Src-family tyrosine kinase Lck. Nature. 1994 Apr 21;368(6473):764–769. doi: 10.1038/368764a0. [DOI] [PubMed] [Google Scholar]
- Elder M. E., Lin D., Clever J., Chan A. C., Hope T. J., Weiss A., Parslow T. G. Human severe combined immunodeficiency due to a defect in ZAP-70, a T cell tyrosine kinase. Science. 1994 Jun 10;264(5165):1596–1599. doi: 10.1126/science.8202712. [DOI] [PubMed] [Google Scholar]
- Emmel E. A., Verweij C. L., Durand D. B., Higgins K. M., Lacy E., Crabtree G. R. Cyclosporin A specifically inhibits function of nuclear proteins involved in T cell activation. Science. 1989 Dec 22;246(4937):1617–1620. doi: 10.1126/science.2595372. [DOI] [PubMed] [Google Scholar]
- Field J., Nikawa J., Broek D., MacDonald B., Rodgers L., Wilson I. A., Lerner R. A., Wigler M. Purification of a RAS-responsive adenylyl cyclase complex from Saccharomyces cerevisiae by use of an epitope addition method. Mol Cell Biol. 1988 May;8(5):2159–2165. doi: 10.1128/mcb.8.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiering S., Northrop J. P., Nolan G. P., Mattila P. S., Crabtree G. R., Herzenberg L. A. Single cell assay of a transcription factor reveals a threshold in transcription activated by signals emanating from the T-cell antigen receptor. Genes Dev. 1990 Oct;4(10):1823–1834. doi: 10.1101/gad.4.10.1823. [DOI] [PubMed] [Google Scholar]
- Flanagan W. M., Corthésy B., Bram R. J., Crabtree G. R. Nuclear association of a T-cell transcription factor blocked by FK-506 and cyclosporin A. Nature. 1991 Aug 29;352(6338):803–807. doi: 10.1038/352803a0. [DOI] [PubMed] [Google Scholar]
- Hall C. G., Sancho J., Terhorst C. Reconstitution of T cell receptor zeta-mediated calcium mobilization in nonlymphoid cells. Science. 1993 Aug 13;261(5123):915–918. doi: 10.1126/science.8346442. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H., Weinert T. A. Checkpoints: controls that ensure the order of cell cycle events. Science. 1989 Nov 3;246(4930):629–634. doi: 10.1126/science.2683079. [DOI] [PubMed] [Google Scholar]
- Holsinger L. J., Spencer D. M., Austin D. J., Schreiber S. L., Crabtree G. R. Signal transduction in T lymphocytes using a conditional allele of Sos. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9810–9814. doi: 10.1073/pnas.92.21.9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley T. R., Amrein K. E., Sefton B. M. Creation and characterization of temperature-sensitive mutants of the lck tyrosine protein kinase. J Virol. 1992 Dec;66(12):7406–7413. doi: 10.1128/jvi.66.12.7406-7413.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchcroft J. E., Harrison M. L., Geahlen R. L. Association of the 72-kDa protein-tyrosine kinase PTK72 with the B cell antigen receptor. J Biol Chem. 1992 Apr 25;267(12):8613–8619. [PubMed] [Google Scholar]
- Karttunen J., Shastri N. Measurement of ligand-induced activation in single viable T cells using the lacZ reporter gene. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3972–3976. doi: 10.1073/pnas.88.9.3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Lippincott-Schwartz J., Bonifacino J. S. The T cell antigen receptor: insights into organelle biology. Annu Rev Cell Biol. 1990;6:403–431. doi: 10.1146/annurev.cb.06.110190.002155. [DOI] [PubMed] [Google Scholar]
- Kolanus W., Romeo C., Seed B. T cell activation by clustered tyrosine kinases. Cell. 1993 Jul 16;74(1):171–183. doi: 10.1016/0092-8674(93)90304-9. [DOI] [PubMed] [Google Scholar]
- Liu J., Farmer J. D., Jr, Lane W. S., Friedman J., Weissman I., Schreiber S. L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell. 1991 Aug 23;66(4):807–815. doi: 10.1016/0092-8674(91)90124-h. [DOI] [PubMed] [Google Scholar]
- Lowell C. A., Soriano P., Varmus H. E. Functional overlap in the src gene family: inactivation of hck and fgr impairs natural immunity. Genes Dev. 1994 Feb 15;8(4):387–398. doi: 10.1101/gad.8.4.387. [DOI] [PubMed] [Google Scholar]
- Luo K., Sefton B. M. Activated lck tyrosine protein kinase stimulates antigen-independent interleukin-2 production in T cells. Mol Cell Biol. 1992 Oct;12(10):4724–4732. doi: 10.1128/mcb.12.10.4724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marth J. D., Cooper J. A., King C. S., Ziegler S. F., Tinker D. A., Overell R. W., Krebs E. G., Perlmutter R. M. Neoplastic transformation induced by an activated lymphocyte-specific protein tyrosine kinase (pp56lck). Mol Cell Biol. 1988 Feb;8(2):540–550. doi: 10.1128/mcb.8.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochly-Rosen D. Localization of protein kinases by anchoring proteins: a theme in signal transduction. Science. 1995 Apr 14;268(5208):247–251. doi: 10.1126/science.7716516. [DOI] [PubMed] [Google Scholar]
- Mustelin T., Coggeshall K. M., Altman A. Rapid activation of the T-cell tyrosine protein kinase pp56lck by the CD45 phosphotyrosine phosphatase. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6302–6306. doi: 10.1073/pnas.86.16.6302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustelin T., Pessa-Morikawa T., Autero M., Gassmann M., Andersson L. C., Gahmberg C. G., Burn P. Regulation of the p59fyn protein tyrosine kinase by the CD45 phosphotyrosine phosphatase. Eur J Immunol. 1992 May;22(5):1173–1178. doi: 10.1002/eji.1830220510. [DOI] [PubMed] [Google Scholar]
- Norbury C., Nurse P. Animal cell cycles and their control. Annu Rev Biochem. 1992;61:441–470. doi: 10.1146/annurev.bi.61.070192.002301. [DOI] [PubMed] [Google Scholar]
- Northrop J. P., Ho S. N., Chen L., Thomas D. J., Timmerman L. A., Nolan G. P., Admon A., Crabtree G. R. NF-AT components define a family of transcription factors targeted in T-cell activation. Nature. 1994 Jun 9;369(6480):497–502. doi: 10.1038/369497a0. [DOI] [PubMed] [Google Scholar]
- Northrop J. P., Ullman K. S., Crabtree G. R. Characterization of the nuclear and cytoplasmic components of the lymphoid-specific nuclear factor of activated T cells (NF-AT) complex. J Biol Chem. 1993 Feb 5;268(4):2917–2923. [PubMed] [Google Scholar]
- O'Keefe S. J., Tamura J., Kincaid R. L., Tocci M. J., O'Neill E. A. FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin. Nature. 1992 Jun 25;357(6380):692–694. doi: 10.1038/357692a0. [DOI] [PubMed] [Google Scholar]
- Pleiman C. M., Clark M. R., Gauen L. K., Winitz S., Coggeshall K. M., Johnson G. L., Shaw A. S., Cambier J. C. Mapping of sites on the Src family protein tyrosine kinases p55blk, p59fyn, and p56lyn which interact with the effector molecules phospholipase C-gamma 2, microtubule-associated protein kinase, GTPase-activating protein, and phosphatidylinositol 3-kinase. Mol Cell Biol. 1993 Sep;13(9):5877–5887. doi: 10.1128/mcb.13.9.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruschy M. N., Spencer D. M., Kapoor T. M., Miyake H., Crabtree G. R., Schreiber S. L. Mechanistic studies of a signaling pathway activated by the organic dimerizer FK1012. Chem Biol. 1994 Nov;1(3):163–172. doi: 10.1016/1074-5521(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Rayter S. I., Woodrow M., Lucas S. C., Cantrell D. A., Downward J. p21ras mediates control of IL-2 gene promoter function in T cell activation. EMBO J. 1992 Dec;11(12):4549–4556. doi: 10.1002/j.1460-2075.1992.tb05556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh M. D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994 Feb 11;76(3):411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Reynolds P. J., Hurley T. R., Sefton B. M. Functional analysis of the SH2 and SH3 domains of the lck tyrosine protein kinase. Oncogene. 1992 Oct;7(10):1949–1955. [PubMed] [Google Scholar]
- Shaw J. P., Utz P. J., Durand D. B., Toole J. J., Emmel E. A., Crabtree G. R. Identification of a putative regulator of early T cell activation genes. Science. 1988 Jul 8;241(4862):202–205. doi: 10.1126/science.3260404. [DOI] [PubMed] [Google Scholar]
- Sloan-Lancaster J., Shaw A. S., Rothbard J. B., Allen P. M. Partial T cell signaling: altered phospho-zeta and lack of zap70 recruitment in APL-induced T cell anergy. Cell. 1994 Dec 2;79(5):913–922. doi: 10.1016/0092-8674(94)90080-9. [DOI] [PubMed] [Google Scholar]
- Spencer D. M., Wandless T. J., Schreiber S. L., Crabtree G. R. Controlling signal transduction with synthetic ligands. Science. 1993 Nov 12;262(5136):1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- Standaert R. F., Galat A., Verdine G. L., Schreiber S. L. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990 Aug 16;346(6285):671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- Stein P. L., Vogel H., Soriano P. Combined deficiencies of Src, Fyn, and Yes tyrosine kinases in mutant mice. Genes Dev. 1994 Sep 1;8(17):1999–2007. doi: 10.1101/gad.8.17.1999. [DOI] [PubMed] [Google Scholar]
- Veillette A., Caron L., Fournel M., Pawson T. Regulation of the enzymatic function of the lymphocyte-specific tyrosine protein kinase p56lck by the non-catalytic SH2 and SH3 domains. Oncogene. 1992 May;7(5):971–980. [PubMed] [Google Scholar]
- Verweij C. L., Geerts M., Aarden L. A. Activation of interleukin-2 gene transcription via the T-cell surface molecule CD28 is mediated through an NF-kB-like response element. J Biol Chem. 1991 Aug 5;266(22):14179–14182. [PubMed] [Google Scholar]
- Weber J. R., Bell G. M., Han M. Y., Pawson T., Imboden J. B. Association of the tyrosine kinase LCK with phospholipase C-gamma 1 after stimulation of the T cell antigen receptor. J Exp Med. 1992 Aug 1;176(2):373–379. doi: 10.1084/jem.176.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Littman D. R. Signal transduction by lymphocyte antigen receptors. Cell. 1994 Jan 28;76(2):263–274. doi: 10.1016/0092-8674(94)90334-4. [DOI] [PubMed] [Google Scholar]
- Weiss A., Stobo J. D. Requirement for the coexpression of T3 and the T cell antigen receptor on a malignant human T cell line. J Exp Med. 1984 Nov 1;160(5):1284–1299. doi: 10.1084/jem.160.5.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T. L., Bolen J. B., Ihle J. N. Hematopoietic cells express two forms of lyn kinase differing by 21 amino acids in the amino terminus. Mol Cell Biol. 1991 May;11(5):2391–2398. doi: 10.1128/mcb.11.5.2391. [DOI] [PMC free article] [PubMed] [Google Scholar]


