Abstract
Pneumonic tularemia is a potentially fatal disease caused by the Category A bioterrorism agent Francisella tularensis. Understanding the pulmonary immune response to this bacterium is necessary for developing effective vaccines and therapeutics. In this study, characterization of immune cell populations in the lungs of mice infected with the type A strain Schu S4 revealed a significant loss in natural killer (NK) cells over time. Since this decline in NK cells correlated with morbidity and mortality, we hypothesized these cells contribute to host defense against Schu S4 infection. Depletion of NK cells prior to Schu S4 challenge significantly reduced IFN-γ and granzyme B in the lung but had no effect on bacterial burden or disease progression. Conversely, increasing NK cell numbers with the anti-apoptotic cytokine IL-15 and soluble receptor IL-15Rα had no significant impact on Schu S4 growth in vivo. A modest decrease in median time to death, however, was observed in live vaccine strain (LVS)-vaccinated mice depleted of NK1.1+ cells and challenged with Schu S4. Therefore, NK cells do not appear to contribute to host defense against acute respiratory infection with type A F. tularensis in vivo, but they play a minor role in protection elicited by LVS vaccination.
Keywords: Francisella tularensis, NK cells, Interleukin-15, Innate Immunity, Vaccination
1. Introduction
The intracellular bacterium Francisella tularensis causes the disease tularemia [1], which can be lethal in 30–60% of untreated individuals after inhalation [2]. Among the four different subspecies, only tularensis (type A) and holarctica (type B) are clinically important [3]. Type A strains are the most virulent and cause approximately 90% of the tularemia cases detected in North America [4]. Furthermore, F. tularensis is a potential bioterrorism agent due to its low infectious dose, ease of aerosolization, and high mortality rate [5]. The development of effective therapeutics and vaccines against F. tularensis depends on a thorough understanding of the pulmonary immune response to this pathogen.
Several studies have characterized the innate immune response elicited following respiratory challenge with the type A F. tularensis strain Schu S4 in mice. After inhalation, this bacterium infects and replicates in multiple cell types in the lung, including alveolar macrophages, airway dendritic cells (DCs), and type II alveolar epithelial cells [6–8]. Although macrophages and DCs typically facilitate clearance of bacterial infections [9], F. tularensis poorly activates these cells [10–12]. F. tularensis-infected macrophages and DCs secrete low levels of proinflammatory cytokines, do not upregulate costimulatory molecules, and are unresponsive to stimulation with Toll-like receptor (TLR) ligands [10–12]. Consequently, F. tularensis replicates exponentially in the lung and disseminates to the spleen and liver two to three days following infection [13]. Inflammatory cell infiltrates comprised predominantly of monocytes and neutrophils are observed in the lung three days post infection [7, 13, 14]. F. tularensis infects neutrophils and inhibits the NADPH oxidase complex, blocking the production of reactive oxygen species [7, 15]. Depleting or enhancing the recruitment of neutrophils has no impact on Schu S4 infection suggesting these cells are not major contributors to host defense [16]. The role of other innate immune cells in type A Francisella infection in vivo has not been investigated.
Natural killer (NK) cells serve two primary functions in combating bacterial infections: perforin-mediated cytolysis and secretion of IFN-γ. While perforin contributes to control of live vaccine strain (LVS) growth in vitro, prf−/− mice remain equally susceptible to F. tularensis [17]. Early during infection, NK cells are recruited to the lung and are the primary source of IFN-γ [18, 19]. Depletion of NK cells results in lower IFN-γ production and shortened mouse survival from lethal pulmonary challenge with LVS [13, 18, 19]. In addition, a number of therapies that reduce mortality associated with pneumonic tularemia are dependent on NK cells. For example, protection mediated by acai polysaccharides and CpG DNA was attributed to inhibition of F. tularensis intramacrophage replication by NK cells [20–22]. NK cells are also required to prolong survival after treatment with recombinant IL-12 and cationic liposome DNA-complexes [23, 24]. While these studies suggest NK cells play a beneficial role in Francisella immunity, the contribution of NK cells during type A pneumonic tularemia has not been directly investigated.
In this study, we evaluated changes in immune cells in the lungs of type A F.tularensis-infected mice. NK cell numbers significantly declined over time, correlating with morbidity and mortality. To assess the role of NK cells in type A Francisella infection, we used two different strategies to modulate NK cell numbers: antibody-mediated depletion and treatment with IL-15 and its receptor (IL-15Rα). Our results indicate that NK cells do not appear to play a major role in host defense against acute respiratory infection with type A F. tularensis, but make a limited contribution in a vaccinated animal.
2. Materials and methods
2.1. Francisella strains and growth conditions
F. tularensis subspecies holarctica LVS was provided by Dr. Karen Elkins (U.S. Food and Drug Administration). F. tularensis subspecies tularensis Schu S4 (strain FSC237, catalog number NR-643) was obtained through the National Institutes of Health (NIH) Biodefense and Emerging Infections Research Resources Repository, National Institute of Allergy and Infectious Diseases. Frozen stock cultures were streaked onto chocolate II agar plates and incubated at 37°C with 5% CO2 for two to three days. These bacteria were then used to inoculate cultures grown in MH broth [Mueller-Hinton broth (Difco) supplemented with 0.1% glucose, 0.025% ferric pyrophosphate (Sigma), and IsoVitaleX (Becton Dickinson)] at 37°C with shaking. All work with Schu S4 was conducted under BSL-3 conditions at the University of Pittsburgh with approval from the CDC Select Agent Program.
2.2. Infection and immunization of mice
All research involving animals was conducted in accordance with animal care and use guidelines, and animal protocols were approved by the Institutional Animal Care and Use Committee. Six- to eight-week old female C57BL/6J mice purchased from Jackson Laboratories (Bar Harbor, ME) were housed in microisolator cages under specific pathogen-free conditions in a biosafety level-3 animal facility. For infections, mice were inoculated intratracheally with ~100 colony forming units (CFU) of LVS or Schu S4 as described previously [11, 25, 26]. For vaccinations, mice were administered ~1000 CFU of LVS intranasally and challenged intratracheally with Schu S4 five weeks later [26].
2.3. Generation of tissue homogenates and enumeration of bacteria
Mice were sacrificed at indicated time points to measure CFU in lungs, spleens, and livers [11, 25, 26]. Spleens and livers were homogenized in TSBc [trypticase soy broth (BD Biosciences) supplemented with 0.1% L-cysteine hydrochloride monohydrate (Fisher)]. Lungs were homogenized in RPMI containing 10% fetal bovine serum (FBS). Blood was collected using a heparin-coated needle and syringe by cardiac puncture. A portion of the organ homogenates and blood were serially diluted and plated onto chocolate II agar plates. Plates were incubated at 37°C at 5% CO2 and individual colonies were enumerated. The limit of detection was 100 CFU per organ (or per ml blood), except the liver, which was 200 CFU. The remaining lung homogenate and blood was centrifuged at 17,000 × g for three min. to remove cells and tissue debris. The resulting supernatant from the lung homogenate was sterile-filtered through 0.2 µm syringe filters, treated with gentamicin (100 µg/ml), and saved for quantification of cytokines and chemokines. Plasma was treated with gentamicin (300 µg/ml) and ciprofloxacin (25 µg/ml) and also saved for quantification of cytokines and chemokines.
2.4. Isolation of lung cells
Lungs were processed using two different enzymatic digestion techniques. For the initial characterization of cellular infiltrates in the lungs of Schu S4-infected mice, lungs were minced and incubated in RPMI (Gibco) supplemented with 1% heat-inactivated FBS, 1 mg/ml collagenase D (Roche), 10 µg/ml DNaseI (USB), and 3 mM CaCl2 for 30 min. at 37°C with shaking (170 rpm). For all subsequent experiments, lungs were minced and incubated in RPMI (Gibco) supplemented with 2.4 mg/ml type I collagenase (Gibco), 20 µg/ml DNase I (USB), and 3 mM CaCl2 for 30 min. at 37°C with shaking (170 rpm). Similar changes in cellular infiltrates were observed over time in Schu S4-infected lungs with both methods, a lthough the latter enzymatic digestion resulted in a two- to three-fold increase in the total number of cells isolated (data not shown). The digested tissue was passed through a 40-µm cell strainer (BD Biosciences) to generate single cell suspensions. Erythrocytes were lysed with ACK (Ammonium-Chloride-Potassium) Lysis Buffer (Gibco) and remaining cells were washed with RPMI. Total live cells were counted using trypan blue exclusion. Lung cells were resuspended in FACS staining buffer [0.1% bovine serum albumin (BSA) and 0.1% sodium azide in phosphate buffered saline (PBS)] prior to flow cytometric analysis.
2.5. Flow cytometry and analysis of lung cells
Individual immune cell populations from the lungs processed above were identified by flow cytometric analysis. Fc receptors were blocked with purified anti-mouse CD16/CD32 (clone 93, eBioscience) for 15 min. on ice. Cells were then stained with the following antibodies at 4°C for 25 min.: FITC (fluorescein isothiocyanate) anti-CD49b (clone DX5, eBioscience), FITC anti-TCR-β (clone H57–597, BD Biosciences), PE (phycoerythrin) anti-Ly-6G (clone 1A8, BD Biosciences), APC (allophycocyanin) anti-F4/80 (clone BM8, eBioscience), PerCP (peridinin-chlorophyll proteins)-Cy5.5 anti-CD11b (clone M1/70, eBioscience), Pacific Blue® anti-NK1.1 (clone PK136, eBioscience), APC-Alexa Fluor750/eFluor™ 450 anti-CD11c (clone N418, eBioscience), APC-eFluor™ 780 anti-TCR-β (clone H57–597), and PerCP-Cy5.5 anti-CD3 (clone 145-2C11, eBioscience). Isotype control antibodies were included in each experiment to confirm specificity. Dead cells were stained using the LIVE/DEAD® Fixable Blue Dead Cell Stain Kit (Invitrogen). Cells were then washed in PBS and fixed in 4% paraformaldehyde for 30 min. at 4°C. For intracellular staining of granzyme B, fixed cells were washed, permeabilized with 0.1% saponin in FACS buffer, and stained with PE-conjugated anti157 granzyme B (clone GB11, Invitrogen). After 25 min., cells were washed and fixed in 2% paraformaldehyde. Samples were collected using a LSRII flow cytometer (BD Biosciences) and analysis gates were set on live cells (negative for LIVE/DEAD® Fixable Blue dye) excluding debris based on forward scatter and side scatter. Approximately 100,000 events were collected for each sample. Data were analyzed using FlowJo Software (Tree Star).
2.6. Cytokine and chemokine assays
Cytokine and chemokine levels in lung supernatants and plasma were determined by ELISA (mouse IFN-γ, R&D Systems) or using the Milliplex 23-plex or 32-plex Mouse Cytokine/Chemokine Panel (Millipore) on a Bio-Plex 200 system (Bio-Rad Laboratories). Analyte concentrations were calculated against the standards using Bio-Plex Manager 5.0 software (Bio-Rad Laboratories, Inc.). Granzyme B levels were measured in lung supernatants by ELISA (eBiosciences). The limits of detection were 31 pg/ml for IFN-γ and 39 pg/ml for granzyme B.
2.7. In vivo depletion of NK cells
Mice were depleted of NK cells using either an anti-asialo GM1 [18] or anti-NK1.1 [27] antibody as described previously with minor modifications. Mice were administered 20 µl of anti-asialo GM1 (Cedarlane) per the manufacturer’s instructions or normal rabbit IgG control (Alpha Diagnostic International Inc.) intranasally (i.n.) and intraperitoneally (i.p.) three days prior to infection with Schu S4 and every three days thereafter until sacrifice. For depletion with anti-NK1.1, mice were administered 200 µg of antibody (Bio X Cell) or mouse IgG2a control (Bio X Cell) i.n. and i.p. three days prior to infection with Schu S4 and every seven days thereafter until sacrifice. Administration of either anti-asialo GM1 or anti-NK1.1 was effective at depleting >90% of the NK cells (DX5/NK1.1+ CD3/TCR-β−) in the lung (data not shown). No significant differences in any other individual immune cell populations were observed in mice administered the isotype control antibodies compared to mice administered PBS (data not shown).
2.8. Administration of IL-15 and IL-15Rα to mice
Mice were treated with a combination of recombinant mouse IL-15 (eBioscience) and mouse IL-15Rα subunit Fc chimera (R&D Systems) as described previously with some modifications [28]. Briefly, IL-15+IL-15Rα complexes were formed by incubating 300 µg of IL-15 and 1.4 mg of IL-15Rα-Fc together in PBS (total volume of 1 ml) at 37°C for 20 min. Samples were then diluted 10-fold in PBS. Each mouse was then administered 50 µl of the IL-15+IL-15Rα (1.5 µg IL-15 and 7 µg IL-15Rα) solution intratracheally (i.t.) one day prior, on the same day, and two days following Schu S4 infection.
2.9. Immunofluorescence staining of lung tissue sections
Lungs were inflated, harvested, and processed as described previously [25]. Deparaffinized tissue sections were blocked with 2.5% BSA in PBS for 30 min. and then probed with rabbit anti-F. tularensis (1:500 dilution; BD Biosciences) overnight at 4°C. As a control, a paired section was probed with normal rabbit IgG (Calbiochem). Sections were washed three times in PBS with 0.2% Tween 20 and then probed with a secondary Alexa Fluor 568 goat anti-rabbit antibody (1:1000 dilution, Invitrogen) for at least 1 hour at 25°C. TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) analysis was performed by first permeabilizing the tissue sections with permeabilization solution (0.1% Triton X-100, 0.1% sodium citrate) for 8 min. on ice. Following permeabilization, the slides were washed three times in PBS and the TUNEL reaction was done using the Fluorescein In Situ Cell Death Detection Kit (Roche). After three PBS washes, sections were then mounted in ProLong Gold antifade reagent containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Invitrogen). Sections were viewed under a Zeiss Axiovert 200 microscope and images were captured at 100× magnification with an equal exposure times within each channel. AxioVision software was used to adjust brightness and contrast uniformly across all images.
3. Results
3.1. Schu S4 reduces NK cell numbers
To evaluate host responses to pneumonic tularemia, we monitored bacterial growth and cell composition in the lungs of F. tularensis-infected mice. Following pulmonary inoculation with comparable doses, LVS and Schu S4 replicated exponentially in the lungs. Both strains grew similarly from one to three days post infection (dpi), but Schu S4 outgrew LVS in the first 24 hours and sustained a higher plateau (Fig. 1A). As bacterial burden increased over time, the total number of live cells also significantly increased in the lungs of LVS-infected mice compared to PBS controls (Fig. 1B). In contrast, the number of viable cells decreased in mice infected with Schu S4, beginning two days post infection (Fig. 1B). After four days, a significant 60–70% reduction in cell numbers was observed (Fig. 1B). To determine whether the loss in viable cells was attributable to the differences in burden, a 100-fold higher dose of LVS was administered to mice and lung cell numbers were evaluated over time. Similar to low dose LVS challenge, the number of live cells increased four dpi with a high dose of LVS compared to PBS (data not shown). The low cell numbers in Schu S4-infected mice corresponded with more apoptotic cells detected in the lung by a TUNEL assay compared to LVS-infected and PBS controls ([29] and data not shown).
Fig. 1. Bacterial replication and changes in immune cell populations in the lung over time following F. tularensis Schu S4 infection.
PBS, LVS, or Schu S4 (~100 CFU) was administered to C57BL/6 mice i.t. (n=2–4 mice/group). At indicated time points, mice were sacrificed and lungs were harvested and homogenized as described in Materials and Methods. (A) Lung homogenates were diluted and plated for CFU enumeration. Data are expressed as mean ± SEM of 4 independent experiments. Some error bars are smaller than the symbols representing each data point. (B) Absolute cell numbers in the lung were enumerated by trypan blue exclusion. Data are expressed as mean ± SEM of at least 4 independent experiments. A total of 9–11 mice per time point were tested for PBS, 12 for LVS, and 14–16 for Schu S4. (C–G) Immune cell populations were identified using standard lineage markers by multi-parameter flow cytometry: neutrophils (Ly6G+, F4/80low), macrophages (F4/80+, Ly-6G−), dendritic cells (CD11chigh, F4/80−), NK cells (DX5+, TCR-β−), and T cells (DX5−, TCR-β+). Total cell numbers for each population were determined by multiplying the percentages derived for each cell population by the total number of lung cells from each individual mouse. Data are expressed as mean ± SEM of two to six independent experiments. A total of 5–6 mice were used per time point for PBS and LVS, and 8–13 for Schu S4. Statistical significance for all experiments was determined by two-way ANOVA, followed by Bonferroni comparison of means (a p<0.05 significant compared to LVS; b p<0.05 significant compared to PBS; c p<0.05 significant compared to day 1; d p<0.05 significant compared to day 3).
Immune cells were then measured over time by flow cytometry. Neutrophils were the predominant cell population recruited to the lungs after LVS infection (approximately 40% of total cells by day four, Fig. 1C), similar to published results [7, 16]. The number of macrophages also increased in the lungs of LVS-infected mice (Fig. 1D). No significant differences in the frequency or absolute number of DCs, T cells, or NK cells were observed in LVS-infected mice compared to PBS controls (Fig. 1E–G). Although neutrophil and macrophage numbers increased up to three dpi with Schu S4 (Fig. 1C–D), similar to previous results [7], their numbers declined sharply from three to four dpi (Fig. 1C–D). Moreover, the number of NK cells and T cells decreased by approximately 90% in the lungs of Schu S4-infected mice four dpi (Fig. 1F–G). In summary, Schu S4 infection reduced cell numbers in the lungs, particularly in the NK cell and T cell populations.
3.2. Schu S4 infection modulates secretion of IFN-γ and granzyme B by NK cell effectors
The loss of NK cells during Schu S4 infection suggested there would be immunological consequences. In LVS infection, NK cells are the major producers of IFN-γ [18]. Mice depleted of NK cells are more susceptible to pulmonary challenge with a sublethal dose of LVS [18]. To evaluate the function of NK cells in Schu S4 infection, cytokines and extracellular granzyme B levels were measured in the lungs of infected mice. In LVS-infected mice, IFN-γ was detected at three dpi in the lung and continued to increase until seven dpi (Fig. 2A, data not shown). In contrast, IFN-γ levels peaked three dpi with Schu S4 and declined four dpi (Fig. 2A). No IFN-γ was detected in PBS controls at any of the time points (Fig. 2A).
Fig. 2. Increase in IFN-γ and extracellular granzyme B levels in the lung of Schu S4-infected mice.
(A–B) At the indicated time points, IFN-γ and granzyme B were measured in lung supernatants from mice treated i.t. with either PBS, LVS, or Schu S4 (100 CFU) using the Milliplex 23-plex Mouse Cytokine/Chemokine Panel (Millipore) on a Bio-Plex system (Bio-Rad Laboratories, Inc.) and an ELISA kit, respectively (n=2–4 mice/group). Data are expressed as mean ± SEM of at least 2 independent experiments. Statistical significance was determined by two-way ANOVA, followed by Bonferroni comparison of means (*** p<0.001 significant compared to PBS). BD = below limits of detection. (C) Analysis of the granzyme B-positive cell populations in the lung of Schu-S4 infected mice three dpi. Cells were stained intracellularly for granzyme B and for surface markers specific for NK (DX5) and T cells (TCR-β). Granzyme B-positive cells that were negative for both DX5 and TCR-β were defined as “other”. Data are expressed as the mean percentage (± SEM) of each cell population that is granzyme B-positive and represent a combination of two independent experiments.
The lytic activity of NK cells was measured by the release of extracellular granzyme B in the lung. Granzyme B was only modestly elevated in the lungs of LVS-infected mice at four dpi compared to PBS controls (Fig. 2B). In contrast, granzyme B levels in Schu S4-infected mice were higher than PBS controls beginning three dpi; and by four dpi (Fig. 2B), approximately three- to four-fold more granzyme B was detected compared to LVS-infected mice (Fig. 2B). Since the accumulation of granzyme B in the lung of Schu S4-infected mice correlated with a decline in NK cells, we were interested in identifying the granzyme B-positive cell populations present in the lung. Lung cells were isolated three dpi from Schu S4-infected mice and stained for intracellular granzyme B. Approximately 78% of the granzyme B-positive cells in the lung were NK cells (Fig. 2C). The remaining granzyme B-positive populations were primarily T cells and DX5− TCR-β− cells (Fig. 2C). Similar results were observed four dpi with Schu S4 (data not shown). Therefore, reduced NK cell numbers in Schu S4-infected mice inversely correlated with the release and accumulation of granzyme B in the lung. IFN-γ levels, however, did not have a clear correlation with loss of NK cells since IFN-γ peaked three dpi when NK cell numbers were beginning to decline.
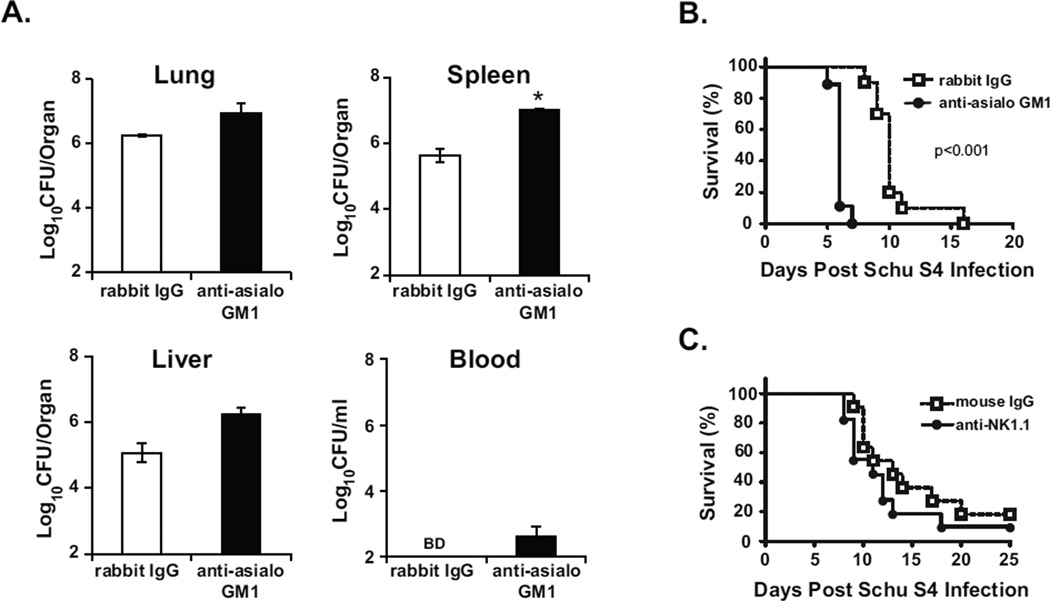
3.3. Depletion of NK cells in Schu S4-infected mice reduces IFN-γ and granzyme B levels in the lung but does not change bacterial burden
Widespread tissue damage and cell death is observed in the lungs of Schu S4-infected mice four dpi [29]. Concomitantly, there was a significant decline in lung NK cells corresponding with reduced IFN-γ and high levels of granzyme B (Fig. 2). To determine whether NK cells have a beneficial or pathologic role in acute Schu S4 infection, NK cells were depleted with an anti-asialo GM1antibody prior to and during infection. Administration of this antibody depleted approximately 90% of the NK cells from the lung (data not shown). Approximately 10-fold less IFN-γ and granzyme B was detected in the lungs of Schu S4-infected mice receiving anti-asialo GM1 compared to an isotype control four dpi (Fig. 3A–B), whereas the treatments had no appreciable effect in mice given PBS (Fig. 3A–B). Based on these data, NK cells were the primary source of IFN-γ and granzyme B in the lung following Schu S4 infection.
Fig. 3. Depletion of NK cells in Schu S4-infected mice reduces IFN-γ and granzyme B in the lung but does not change bacterial burdens.
Mice (n=4 per group) were administered anti-asialo GM1 or normal rabbit IgG i.n. and i.p three days prior to i.t. infection with Schu S4 (100 CFU) and every three days thereafter. PBS-treated mice (n=4 per group) administered either anti-asialo GM1 or rabbit IgG served as controls. Four dpi, mice were sacrificed and lungs, spleens, livers, and blood were harvested and processed as described in the Materials and Methods. (A–B) IFN-γ and granzyme B were measured in lung supernatants by ELISA. (C) Organ homogenates and blood were diluted and plated for CFU enumeration. Data are expressed as mean ± SEM of 3 independent experiments. Statistical significance was determined by two-way ANOVA, followed by Bonferroni comparison of means (** p<0.01, *** p<0.001 significant compared to rabbit IgG). BD = below limits of detection.
We next investigated the effect of NK cell depletion on the course of Schu S4 infection. Despite substantial effects on IFN-γ and granzyme B (Fig. 3A–B), no differences in bacterial burdens were seen in Schu S4-infected mice treated with the anti-asialo GM1 antibody compared to isotype controls four dpi (Fig. 3C). Similar results were observed two dpi (data not shown). In addition, Schu S4-infected mice depleted of NK cells exhibited similar clinical signs of illness and weight loss (14% for isotype and 16% for anti-asialo GM1 four dpi) compared to infected controls. Therefore, NK cells did not have a demonstrable impact on host defense against acute Schu S4 infection.
3.4. Contribution of NK cells to host resistance to Schu S4 challenge in LVS-vaccinated mice
In addition to their role in innate immunity, NK cells also influence adaptive immune responses [30]. Though NK cell depletion had little effect on acute infection, we considered the possibility that NK cells might contribute to host resistance to Schu S4 infection in LVS-vaccinated mice. To assess the role of NK cells in protection elicited by LVS, NK cells were depleted from vaccinated mice with antibodies prior to Schu S4 infection. Four dpi, a 0.5–1.5 log increase in Schu S4 burden was measured in all organs of vaccinated mice that received anti-asialo GM1 compared to vaccinated mice treated with control rabbit IgG (Fig. 4A). We hypothesized that greater Schu S4 burdens would translate to reduced survival after NK cell depletion. Anti-asialo GM1 treatment caused a significant reduction in survival of LVS-vaccinated mice compared to controls, where the median time to death (MTD) shifted from 10 to 6 days (Fig 4B). These data demonstrate that anti-asialo GM1 treatment increased infection severity and reduced survival of LVS-vaccinated mice following Schu S4 challenge.
Fig. 4. Contribution of NK cells in LVS-mediated immunity to Schu S4 challenge.
Mice were vaccinated intranasally with LVS (1000 CFU) or administered PBS as a control (naïve). Five weeks later, mice were challenged i.t with Schu S4 (100 CFU). (A) Treatment of vaccinated mice with anti-asialo GM1 increases bacterial burdens in the organs and blood following Schu S4 infection. LVS-vaccinated mice (n=4–5 mice per group) were administered anti-asialo GM1 or normal rabbit IgG prior to infection with Schu S4 as described in the Materials and Methods. Four days post Schu S4 infection, mice were sacrificed and the designated organs and blood were processed, diluted, and plated for CFU enumeration. Data are expressed as mean ± SEM of two independent experiments. Statistical significance was determined by two-way ANOVA, followed by Bonferroni comparison of means (* p<0.05 significant compared to rabbit IgG). BD = below limits of detection. (B) Reduced survival of LVS-vaccinated mice treated with anti-asialo GM1and infected with Schu S4. The data are combined from two independent experiments (n=5 mice per group). p<0.001, log-rank test. (C) NK cells play a minor role in immunity to Schu S4 infection in LVS-vaccinated mice. LVS632 vaccinated mice (n=5–6 mice per group) were administered anti-NK1.1 or mouse IgG prior to infection with Schu S4 as described in the Materials and Methods. Survival was monitored over time. The data are combined from two independent experiments. Statistical significance was reached in only one experiment (p<0.05, log-rank test).
Although asialo-GM1 is predominantly expressed on NK cells, this marker is also expressed on a subset of other immune cell populations such as CD8+ T cells and basophils ([31, 32] and data not shown). To test the specificity of the results obtained with anti-asialo GM1, we used an alternative depletion strategy with a different antibody, anti-NK1.1, prior to challenge with Schu S4. Compared to the four day difference in survival observed with anti-asialo GM-treated vaccinated mice and isotype controls, vaccinated mice treated with anti-NK1.1 succumbed to Schu S4 challenge only one to two days earlier than mouse IgG controls (Fig. 4C). This difference in survival between the two depletion strategies suggested NK cells had a minor role in LVS-mediated protective immunity to Schu S4 challenge.
3.5. Enhanced survival of lymphocytes and reduced systemic cytokine production induced by IL-15+IL-15Rα does not improve host resistance to acute Schu S4 infection
Depletion of NK cells failed to exacerbate or ameliorate acute Schu S4 infection. Augmenting NK cell numbers and activation, however, may improve host defenses since these cells are major producers of IFN-γ [18]. The anti-apoptotic cytokine IL-15 plays a critical role in the development, homeostasis, and function of NK cells and enhances the proliferation of NK cells in vivo when given with a soluble form of its receptor (IL-15Rα) [33–36]. IL-15+IL-15Rα treatment reduces NK cell apoptosis, increases expression of IFN-γ, and improves survival in two murine models of sepsis [28]. Since Schu S4 infection also has features of sepsis, we tested the effects of IL-15+IL-15Rα in this model.
Four dpi, intratracheal IL-15+IL-15Rα treatment increased viable lung cells of Schu S4-infected mice by two-fold compared to Schu S4-infected controls treated with PBS (Fig. 5A). A similar increase in lung cells was observed in uninfected mice receiving IL-15+IL-15Rα (Fig. 5A). Among the lung immune cells evaluated, no significant differences in the frequency or absolute number of macrophages and neutrophils were observed in Schu S4-infected mice after IL-15+IL-15Rα treatment (Fig 5B–C). Although IL-15+IL-15Rα significantly increased the frequency of DCs in the lung of uninfected controls, there was no effect on DCs in Schu S4-infected mice (Fig. 5D). More importantly, IL-15+IL-15Rα treatment significantly increased the frequency of NK cells and T cells in the lungs of both uninfected and Schu S4-infected mice by 70–80% (Fig. 5E–F). Therefore, IL-15+IL-15Rα treatment enhanced the number of viable cells during Schu S4 infection.
Fig. 5. Changes in lung immune cell populations and systemic cytokine and chemokine production following IL-15+IL-15Rα treatment of Schu S4-infected mice.
Mice (n=4 per group) were administered PBS or IL-15+IL-15Rα i.t. one day prior, on the same day, and two days following i.t. Schu S4 infection (100 CFU). Uninfected (Uninf.) mice (PBS-treated, n=4 per group) administered PBS or IL-15+IL-15Rα served as controls. Mice were sacrificed four dpi and lungs were processed as described in Materials and Methods. (A) Absolute cell numbers in the lungs were enumerated by trypan blue exclusion. (B–F) Changes in individual immune cell populations were evaluated by flow cytometric analysis: neutrophils (Ly6G+, F4/80low), macrophages (F4/80+, Ly-6G−), dendritic cells (CD11chigh, F4/80−), NK cells (DX5+, TCR-β−), and T cells (DX5−, TCR-β+). (G) Lung supernatants were assessed for multiple cytokines and chemokines using the Milliplex 32-plex Mouse Cytokine/Chemokine Panel (Millipore) on a Bio-Plex system (Bio-Rad Laboratories, Inc.). The cytokines shown here were previously demonstrated to be modulated by IL-15+IL-15Rα in other models of sepsis [28]. All other cytokine and chemokine values can be found in Supp. Table 1. Data are expressed as mean ± SEM of two independent experiments. Statistical significance was determined by two-way ANOVA for each cell type and analyte comparing the effects of infection and treatment, followed by Bonferroni comparison of means (*p<0.05, **p<0.01, significant compared to PBS treatment).
We next tested whether IL-15+IL-15Rα treatment altered systemic cytokines and chemokines after Schu S4 infection. In other models of sepsis, IL-15+IL-15α modulated the levels of circulating IFN-γ, IL-6, and TNF-α [28]. Analyzing plasma by Luminex, there was a statistically significant reduction in IFN-γ and IL-6 in Schu S4-infected mice treated with IL-15+IL-15Rα compared to PBS-treated controls (Fig. 5G). TNF-α levels in Schu S4-infected mice remained unchanged in response to IL-15+IL-15Rα treatment (Fig. 5G). In addition to IFN-γ and IL-6, ten other cytokines and chemokines were significantly downregulated in Schu S4-infected mice treated with IL-15+IL-15Rα compared to PBS (Supp. Table 1). No statistically significant differences in chemokine and cytokine concentrations were detected in uninfected mice treated with IL-15+IL-15Rα and PBS (Supp. Table 1). Therefore, IL-15+IL-15Rα reduced inflammatory mediators in the bloodstream of Schu S4-infected mice.
The effect of IL-15+IL-15Rα on bacterial burdens in Schu S4-infected mice was then measured. Despite changes in immune cells and modulation of cytokines, IL-15+IL-15Rα had no impact on bacterial numbers measured four dpi (Fig. 6A). In addition, IL-15+IL-15Rα did not significantly alter the progression of disease in Schu S4-infected mice based on clinical signs and weight loss (17% for PBS-treated and 16% for IL-15+IL-15Rα-treated four dpi). Administration of IL-15+IL-15Rα by different routes, either intraperitoneally or both intraperitoneally and intratracheally, did not affect bacterial burdens or survival following Schu S4 infection (data not shown). Therefore, IL-15+IL-15Rα treatment did not improve host resistance to pulmonary Schu S4 infection.
Fig. 6. Bacterial burden and TUNEL staining following IL-15+IL-15Rα treatment of Schu S4-infected mice.
Mice were administered PBS or IL-15+IL-15Rα and infected with Schu S4 as described in the legend to Fig. 5. (A) Bacterial burdens in the organs and blood of Schu S4-infected mice treated with IL-15+IL-15Rα four dpi (n=4 mice per group). Data are expressed as mean ± SEM and are representative of two independent experiments. (B) Immunofluorescence microscopy of lung sections from uninfected and Schu S4-infected mice treated with IL-15+IL-15Rα. Four dpi, lungs were harvested, fixed in formalin, and embedded in paraffin. Deparaffinized lung sections were probed with rabbit anti-F. tularensis followed by a secondary Alexa Fluor 568 goat anti-rabbit antibody. An In Situ Cell Death Detection Kit (Roche) was used for the TUNEL assay. DNA was stained with DAPI. The color merged images (red, F. tularensis; green, TUNEL; blue, DAPI) were captured with an equal fluorescence exposure time within each channel. Lung sections from 3–4 mice per group from two independent experiments were stained. The scale bars in the upper left hand corners of the images represent a length of 100 µm.
Although IL-15+IL-15Rα improved the absolute number of viable cells present in the lungs of Schu S4-infected mice, there was still a 60–70% loss in viable lung cells after Schu S4 infection irrespective of treatment group (Fig. 5A). This suggested IL-15+IL-15Rα was not limiting the widespread cell death that occurred in the lungs of Schu S4-infected mice. To investigate this further, lung sections from treated and untreated Schu S4-infected mice were probed for apoptotic cells. As observed previously [29], robust TUNEL staining was localized around regions of infection in the lung of Schu S4-infected mice four dpi (Fig. 6B). A similar pattern was observed in Schu S4-infected mice that were treated with IL-15+IL-15Rα that included peribronchial (Fig. 6B) and parenchymal staining (data not shown). In regions with no bacterial antigen staining, a low frequency of TUNEL+ cells was detected (data not shown). Limited TUNEL staining was also seen in uninfected mice (Fig. 6B). These results corroborated the loss in lung cells observed by flow cytometric analysis and suggest that the inability of IL-15+IL-15Rα to limit cell death in the lung may contribute to its poor effectiveness as a treatment for type A F. tularensis.
4. Discussion
NK cells can promote or antagonize host resistance to bacterial pathogens [37–40]. In studies with LVS, NK cells are important for the early control of infection through the secretion of IFN-γ [13, 18, 19]. We began investigating NK cells in Schu S4 infection because of their role in LVS immunity and because of the marked changes observed after pulmonary challenge (Fig. 1F). Similar to neutrophils [16], modulation of NK cells did not have a demonstrable effect on Schu S4 infection in vivo. Neither depletion with antibodies nor enhanced NK cell numbers via treatment with IL-15+IL-15Rα had an impact on bacterial burden or the progression of disease following Schu S4 challenge. Our results indicate NK cells do not appear to contribute to the control of acute type A Francisella infection.
While NK cell numbers remained constant in LVS-infected mice, a significant drop in NK cells was observed in Schu S4-infected mice (Fig. 1F). Since loss of other lymphocytes was observed after Schu S4 infection (Fig. 1G and [29]), it was not surprising that NK cells also declined over time (Fig. 1F). Intrinsic and extrinsic signals activate caspase 3 and induce T cell apoptosis during sepsis [41]. It is unlikely, however, that these pathways contribute to NK and T cell death in Schu S4-infected mice because few caspase 3-positive cells are detected in lungs following challenge [12, 42]. It is also possible that NK cell death is due to direct infection. Preliminary in vitro studies suggest Schu S4 can invade but not replicate in NK cells (data not shown). Therefore, direct cell invasion may contribute to NK cell loss in vivo.
Different results were observed when NK cells were depleted with anti-asialo GM1 or anti-NK1.1 in the vaccination/challenge model (Fig. 4B–C). Anti-asialo GM1 treatment reduced survival of LVS-vaccinated mice challenged with Schu S4. An explanation for this effect is a partial loss in CD8+ T cells in anti-asialo GM1-treated mice. Although asialo GM1 is expressed predominantly on NK cells, it is also detected on a subpopulation of NK T cells, activated CD8+ T cells, gamma-delta T cells, and basophils [32, 43]. While no off-target effects of anti-asialo GM1 were observed in naive mice, there was a 60% reduction in the number of CD8+ T cells in vaccinated mice treated with anti-asialo GM1 compared to isotype controls four days post Schu S4 infection (data not shown). Previous work by Conlan et al. demonstrated that depletion of CD8+ T cells significantly reduces survival from secondary challenge with type A Francisella [44]. Anti-asialo GM1, therefore, may have depleted Francisella-immune CD8+ T cells making mice more susceptible to challenge with Schu S4. Additionally, the biological impact of anti-NK1.1 treatment, which does not affect CD8+ T cells, was small (Fig. 4C). Together, these results suggest NK cells minimally contribute to LVS-mediated protective immunity.
While NK cell depletion failed to alter acute Schu S4 infection, we also tested the effect of increasing NK cell numbers. IL-15+IL-15Rα treatment had a significant effect on survival in a mouse model of cecal ligation and puncture (CLP) and Pseudomonas aeruginosa pneumonia [28]. However, no difference in bacterial burden or disease severity was observed between IL-15+IL-15Rα-treated and PBS-treated mice infected with Schu S4 (Fig. 6A). There are several possible explanations for the effects of IL-15+IL-15Rα in these different sepsis models. Although serum IFN-γ levels increased with IL-15+IL-15Rα in CLP mice [28], IFN-γ decreased following IL-15+IL-15Rα treatment in Schu S4-infected mice (Fig. 5G). To compensate for this, we also treated Schu S4-infected mice with recombinant IFN-γ along with IL-15+IL-15Rα. However, no significant difference in bacterial burden or disease progression was observed in Schu S4-infected mice treated with IFN-γ+ IL-15+IL-15Rα compared to PBS controls (data not shown).
Other cytokines may also contribute to the limited effect of IL-15+ IL-15Rα in pneumonic tularemia. Systemic levels of TNF-α and IL-6 were significantly lower in CLP mice treated with IL-15+IL-15Rα [28], but only IL-6 was significantly reduced in IL-15+IL-15Rα-treated Schu S4-infected mice (Fig. 5G and Supp. Table 1). Because high circulating levels of TNF-α are associated with mortality in some models of sepsis [45], persistently elevated TNF-α may worsen Schu S4-infected mice despite IL-15+IL-15Rα therapy.
IL-15+IL-15Rα also prevents the apoptotic depletion of lymphocytes and DCs in CLP mice [28]. However, a 60–70% loss in viable lung cells was still observed in IL-15+IL-15Rα-treated mice infected with Schu S4 compared to uninfected controls (Fig. 5A). The absolute number of DCs, T cells, and NK cells also declined at a similar rate (Fig. 5D–F). TUNEL staining of lung tissue sections from both PBS and IL-15+IL-15Rα-treated mice infected with Schu S4 confirmed that cell death was not limited by cytokine treatment (Fig. 6B). These results suggest the mechanism of cell death in Schu S4-infected mice may differ from the Bim and PUMA423 dependent apoptosis in CLP mice [28]. In summary, IL-15+IL-15Rα has different effects on systemic cytokine production and cell death in two independent sepsis models: CLP and pneumonic tularemia. This suggests IL-15+IL-15Rα may not be generally applicable to treat sepsis of different etiologies.
Supplementary Material
Total live cells were gated based on forward and side scatter characteristics and exclusion of the LIVE/DEAD® Fixable Blue dye. Macrophages and neutrophils were identified based on F4/80 and Ly-6G expression. F4/80−Ly-6G− cells were divided into T cells, NK cells, and dendritic cells based on CD11c, TCR-β, and CD49b (DX5) expression.
Total live cells were gated based on forward and side scatter characteristics and exclusion of the LIVE/DEAD® Fixable Blue dye. Next, granzyme B+ cells were gated and divided into NK cells, T cells, and “other” based on TCR-β and CD49b (DX5) expression.
Acknowledgements
We thank Dr. Karen Elkins for F. tularensis LVS. F. tularensis subsp. tularensis Schu S4 was obtained through the NIH Biodefense and Emerging Infections Research Resources Repository (NIAID; FSC237 and NR-643). We are grateful to the laboratories of Dr. JoAnne Flynn and Dr. Robert Hendricks for providing flow cytometry staining protocols, the laboratory of Dr. Ted Ross for technical assistance with lung cell isolations and the Bio-Plex 200 system, and the laboratory of Dr. Angus Thomson for providing the mouse IL-6 ELISA kit. We also thank Tim Sturgeon for assistance with flow cytometry. This work was funded by the National Institutes of Health grants AI057168 and AI050018 and institutional funding from the Department of Microbiology and Molecular Genetics. DMS is a recipient of T32 AI060525, "Immunology of Infectious Disease.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Oyston PC. Francisella tularensis: unravelling the secrets of an intracellular pathogen. J. Med. Microbiol. 2008;57:921–930. doi: 10.1099/jmm.0.2008/000653-0. [DOI] [PubMed] [Google Scholar]
- 2.Dennis DT, Inglesby TV, Henderson DA, Bartlett JG, Ascher MS, Eitzen E, Fine AD, Friedlander AM, Hauer J, Layton M, Lillibridge SR, McDade JE, Osterholm MT, O'Toole T, Parker G, Perl TM, Russell PK, Tonat K. Tularemia as a biological weapon: medical and public health management. JAMA. 2001;285:2763–2773. doi: 10.1001/jama.285.21.2763. [DOI] [PubMed] [Google Scholar]
- 3.Molins CR, Delorey MJ, Yockey BM, Young JW, Sheldon SW, Reese SM, Schriefer ME, Petersen JM. Virulence differences among Francisella tularensis subsp tularensis clades in mice. PLoS One. 2010;5:e10205. doi: 10.1371/journal.pone.0010205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choi E. Tularemia and Q fever. Med. Clin. North Am. 2002;86:393–416. doi: 10.1016/s0025-7125(03)00094-4. [DOI] [PubMed] [Google Scholar]
- 5.McLendon MK, Apicella MA, Allen LA. Francisella tularensis: taxonomy, genetics, and Immunopathogenesis of a potential agent of biowarfare. Annu. Rev. Microbiol. 2006;60:167–185. doi: 10.1146/annurev.micro.60.080805.142126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall JD, Craven RR, Fuller JR, Pickles RJ, Kawula TH. Francisella tularensis replicates within alveolar type II epithelial cells in vitro and in vivo following inhalation. Infect. Immun. 2007;75:1034–1039. doi: 10.1128/IAI.01254-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, Frelinger JA, Kawula TH. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect. Immun. 2008;76:5843–5852. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosio CM, Dow SW. Francisella tularensis induces aberrant activation of pulmonary dendritic cells. J. Immunol. 2005;175:6792–6801. doi: 10.4049/jimmunol.175.10.6792. [DOI] [PubMed] [Google Scholar]
- 9.Lambrecht BN, Prins JB, Hoogsteden HC. Lung dendritic cells and host immunity to infection. Eur. Respir. J. 2001;18:692–704. [PubMed] [Google Scholar]
- 10.Carlson PE, Jr, Horzempa J, O'Dee DM, Robinson CM, Neophytou P, Labrinidis A, Nau GJ. Global transcriptional response to spermine, a component of the intramacrophage environment, reveals regulation of Francisella gene expression through insertion sequence elements. J. Bacteriol. 2009;191:6855–6864. doi: 10.1128/JB.00995-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo BC, Horzempa J, O'Dee DM, Schmitt DM, Brown MJ, Carlson PE, Jr, Xavier RJ, Nau GJ. A Francisella tularensis locus required for spermine responsiveness is necessary for virulence. Infect. Immun. 2011;79:3665–3676. doi: 10.1128/IAI.00135-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bosio CM, Bielefeldt-Ohmann H, Belisle JT. Active suppression of the pulmonary immune response by Francisella tularensis Schu4. J. Immunol. 2007;178:4538–4547. doi: 10.4049/jimmunol.178.7.4538. [DOI] [PubMed] [Google Scholar]
- 13.Metzger DW, Bakshi CS, Kirimanjeswara G. Mucosal immunopathogenesis of Francisella tularensis. Ann. N. Y. Acad. Sci. 2007;1105:266–283. doi: 10.1196/annals.1409.007. [DOI] [PubMed] [Google Scholar]
- 14.Conlan JW, Chen W, Shen H, Webb A, KuoLee R. Experimental tularemia in mice challenged by aerosol or intradermally with virulent strains of Francisella tularensis: bacteriologic and histopathologic studies. Microb. Pathog. 2003;34:239–248. doi: 10.1016/s0882-4010(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 15.McCaffrey RL, Schwartz JT, Lindemann SR, Moreland JG, Buchan BW, Jones BD, Allen LA. Multiple mechanisms of NADPH oxidase inhibition by type A and type B Francisella tularensis. J. Leukoc. Biol. 2010;88:791–805. doi: 10.1189/jlb.1209811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.KuoLee R, Harris G, Conlan JW, Chen W. Role of neutrophils and NADPH phagocyte oxidase in host defense against respiratory infection with virulent Francisella tularensis in mice. Microbes Infect. 2011;13:447–456. doi: 10.1016/j.micinf.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 17.Sanapala S, Yu JJ, Murthy AK, Li W, Guentzel MN, Chambers JP, Klose KE, Arulanandam BP. Perforin- and Granzyme-Mediated Cytotoxic Effector Functions Are Essential for Protection against Francisella tularensis following Vaccination by the Defined F. tularensis subsp. novicida {Delta}fopC Vaccine Strain. Infect. Immun. 2012;80:2177–2185. doi: 10.1128/IAI.00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez MC, Duckett NS, Baron SD, Metzger DW. Early activation of NK cells after lung infection with the intracellular bacterium, Francisella tularensis LVS. Cell Immunol. 2004;232:75–85. doi: 10.1016/j.cellimm.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Bokhari SM, Kim KJ, Pinson DM, Slusser J, Yeh HW, Parmely MJ. NK cells and gamma interferon coordinate the formation and function of hepatic granulomas in mice infected with the Francisella tularensis live vaccine strain. Infect. Immun. 2008;76:1379–1389. doi: 10.1128/IAI.00745-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elkins KL, Rhinehart-Jones TR, Stibitz S, Conover JS, Klinman DM. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 1999;162:2291–2298. [PubMed] [Google Scholar]
- 21.Elkins KL, Colombini SM, Krieg AM, De Pascalis R. NK cells activated in vivo by bacterial DNA control the intracellular growth of Francisella tularensis LVS. Microbes Infect. 2009;11:49–56. doi: 10.1016/j.micinf.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Skyberg JA, Rollins MF, Holderness JS, Marlenee NL, Schepetkin IA, Goodyear A, Dow SW, Jutila MA, Pascual DW. Nasal Acai polysaccharides potentiate innate immunity to protect against pulmonary Francisella tularensis and Burkholderia pseudomallei Infections. PLoS Pathog. 2012;8:e1002587. doi: 10.1371/journal.ppat.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duckett NS, Olmos S, Durrant DM, Metzger DW. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 2005;73:2306–2311. doi: 10.1128/IAI.73.4.2306-2311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troyer RM, Propst KL, Fairman J, Bosio CM, Dow SW. Mucosal immunotherapy for protection from pneumonic infection with Francisella tularensis. Vaccine. 2009;27:4424–4433. doi: 10.1016/j.vaccine.2009.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Horzempa J, O'Dee DM, Shanks RM, Nau GJ. Francisella tularensis DeltapyrF mutants show that replication in nonmacrophages is sufficient for pathogenesis in vivo. Infect. Immun. 2010;78:2607–2619. doi: 10.1128/IAI.00134-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitt DM, O'Dee DM, Horzempa J, Carlson PE, Jr, Russo BC, Bales JM, Brown MJ, Nau GJ. A Francisella tularensis live vaccine strain that improves stimulation of antigen-presenting cells does not enhance vaccine efficacy. PLoS One. 2012;7:e31172. doi: 10.1371/journal.pone.0031172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thatte A, DeWitte-Orr SJ, Lichty B, Mossman KL, Ashkar AA. A critical role for IL-15 in TLR-mediated innate antiviral immunity against genital HSV-2 infection. Immunol. Cell Biol. 2011;89:663–669. doi: 10.1038/icb.2011.7. [DOI] [PubMed] [Google Scholar]
- 28.Inoue S, Unsinger J, Davis CG, Muenzer JT, Ferguson TA, Chang K, Osborne DF, Clark AT, Coopersmith CM, McDunn JE, Hotchkiss RS. IL-15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J. Immunol. 2010;184:1401–1409. doi: 10.4049/jimmunol.0902307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sharma J, Mares CA, Li Q, Morris EG, Teale JM. Features of sepsis caused by pulmonary infection with Francisella tularensis Type A strain. Microb. Pathog. 2011;51:39–47. doi: 10.1016/j.micpath.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall LJ, Clare S, Dougan G. NK cells influence both innate and adaptive immune responses after mucosal immunization with antigen and mucosal adjuvant. J. Immunol. 2010;184:4327–4337. doi: 10.4049/jimmunol.0903357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee U, Santa K, Habu S, Nishimura T. Murine asialo GM1+CD8+ T cells as novel interleukin-12-responsive killer T cell precursors. Jpn. J. Cancer Res. 1996;87:429–432. doi: 10.1111/j.1349-7006.1996.tb00241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishikado H, Mukai K, Kawano Y, Minegishi Y, Karasuyama H. NK cell-depleting anti-asialo GM1 antibody exhibits a lethal off-target effect on basophils in vivo. J. Immunol. 2011;186:5766–5771. doi: 10.4049/jimmunol.1100370. [DOI] [PubMed] [Google Scholar]
- 33.Bulfone-Paus S, Ungureanu D, Pohl T, Lindner G, Paus R, Ruckert R, Krause H, Kunzendorf U. Interleukin-15 protects from lethal apoptosis in vivo. Nat. Med. 1997;3:1124–1128. doi: 10.1038/nm1097-1124. [DOI] [PubMed] [Google Scholar]
- 34.Oh S, Perera LP, Terabe M, Ni L, Waldmann TA, Berzofsky JA. IL-15 as a mediator of CD4+ help for CD8+ T cell longevity and avoidance of TRAIL-mediated apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5201–5206. doi: 10.1073/pnas.0801003105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stonier SW, Schluns KS. Trans-presentation: a novel mechanism regulating IL-15 delivery and responses. Immunol. Lett. 2010;127:85–92. doi: 10.1016/j.imlet.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc. Natl. Acad. Sci. U. S. A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Badgwell B, Parihar R, Magro C, Dierksheide J, Russo T, Carson WE., 3rd Natural killer cells contribute to the lethality of a murine model of Escherichia coli infection. Surgery. 2002;132:205–212. doi: 10.1067/msy.2002.125311. [DOI] [PubMed] [Google Scholar]
- 38.Sherwood ER, Enoh VT, Murphey ED, Lin CY. Mice depleted of CD8+ T and NK cells are resistant to injury caused by cecal ligation and puncture. Lab. Invest. 2004;84:1655–1665. doi: 10.1038/labinvest.3700184. [DOI] [PubMed] [Google Scholar]
- 39.Le-Barillec K, Magalhaes JG, Corcuff E, Thuizat A, Sansonetti PJ, Phalipon A, Di Santo JP. Roles for T and NK cells in the innate immune response to Shigella flexneri. J. Immunol. 2005;175:1735–1740. doi: 10.4049/jimmunol.175.3.1735. [DOI] [PubMed] [Google Scholar]
- 40.Feng CG, Kaviratne M, Rothfuchs AG, Cheever A, Hieny S, Young HA, Wynn TA, Sher A. NK cell-derived IFN-gamma differentially regulates innate resistance and neutrophil response in T cell-deficient hosts infected with Mycobacterium tuberculosis. J. Immunol. 2006;177:7086–7093. doi: 10.4049/jimmunol.177.10.7086. [DOI] [PubMed] [Google Scholar]
- 41.Hotchkiss RS, Coopersmith CM, Karl IE. Prevention of lymphocyte apoptosis--a potential treatment of sepsis? Clin. Infect. Dis. 2005;41(Suppl. 7):S465–S469. doi: 10.1086/431998. [DOI] [PubMed] [Google Scholar]
- 42.Parmely MJ, Fischer JL, Pinson DM. Programmed cell death and the pathogenesis of tissue injury induced by type A Francisella tularensis. FEMS Microbiol. Lett. 2009;301:1–11. doi: 10.1111/j.1574-6968.2009.01791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trambley J, Bingaman AW, Lin A, Elwood ET, Waitze SY, Ha J, Durham MM, Corbascio M, Cowan SR, Pearson TC, Larsen CP. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wayne Conlan J, Shen H, Kuolee R, Zhao X, Chen W. Aerosol-but not intradermal-immunization with the live vaccine strain of Francisella tularensis protects mice against subsequent aerosol challenge with a highly virulent type A strain of the pathogen by an alphabeta T cell- and interferon gamma- dependent mechanism. Vaccine. 2005;23:2477–2485. doi: 10.1016/j.vaccine.2004.10.034. [DOI] [PubMed] [Google Scholar]
- 45.Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N. Engl. J. Med. 2003;348:138–150. doi: 10.1056/NEJMra021333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Total live cells were gated based on forward and side scatter characteristics and exclusion of the LIVE/DEAD® Fixable Blue dye. Macrophages and neutrophils were identified based on F4/80 and Ly-6G expression. F4/80−Ly-6G− cells were divided into T cells, NK cells, and dendritic cells based on CD11c, TCR-β, and CD49b (DX5) expression.
Total live cells were gated based on forward and side scatter characteristics and exclusion of the LIVE/DEAD® Fixable Blue dye. Next, granzyme B+ cells were gated and divided into NK cells, T cells, and “other” based on TCR-β and CD49b (DX5) expression.