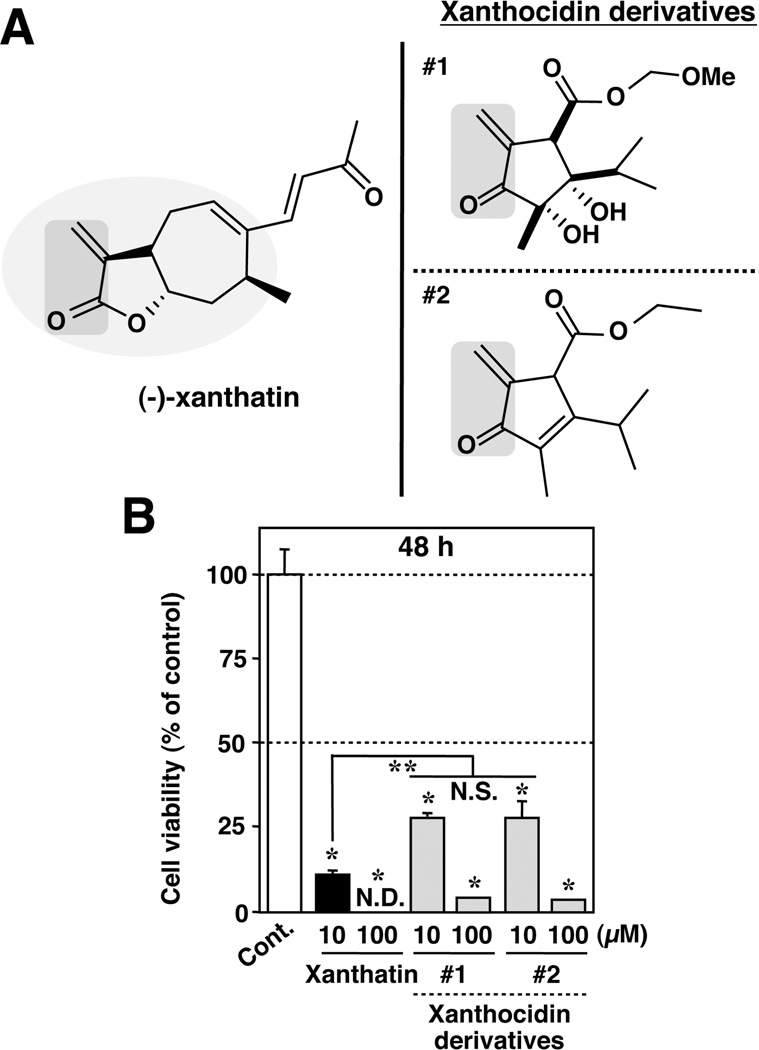
Figure 7.
(−)-Xanthatin-mediated antitumor effects minimally require its exo-methylene-coupled carbonyl moiety. (A) Chemical structures of (−)-xanthatin (left panel) and two xanthocidin derivatives (right panel, #1 and #2). The exo-methylene-coupled carbonyl moiety in the structures is indicated with a gray inclusion. Me, − CH3 (methyl group). (B) MDA-MB-231 cells were exposed for 48 h to the indicated concentrations (10, 100 µM) of the compounds listed in panel A. Data are expressed as the percent of the vehicle-treated group (control) and represent the mean ± SD (n = 5). *Significantly different (p < 0.05) from the control. **Significantly different (p < 0.05) from the 10 µM (−)-xanthatin-treated group. N.D., not detectable (because of complete cell death). N.S., not significant.