Abstract
Magnetic resonance imaging (MRI), frequently with contrast enhancement, is the preferred imaging modality for many indications in children. Practice varies widely between centers, reflecting the rapid pace of change and the need for further research. Guide-line changes, for example on contrast-medium choice, require continued practice reappraisal. This article reviews recent developments in pediatric contrast-enhanced MRI and offers recommendations on current best practice. Nine leading pediatric radiologists from internationally recognized radiology centers convened at a consensus meeting in Bordeaux, France, to discuss applications of contrast-enhanced MRI across a range of indications in children. Review of the literature indicated that few published data provide guidance on best practice in pediatric MRI. Discussion among the experts concluded that MRI is preferred over ionizing-radiation modalities for many indications, with advantages in safety and efficacy. Awareness of age-specific adaptations in MRI technique can optimize image quality. Gadolinium-based contrast media are recommended for enhancing imaging quality. The choice of most appropriate contrast medium should be based on criteria of safety, tolerability, and efficacy, characterized in age-specific clinical trials and personal experience.
Keywords: magnetic resonance imaging, contrast-enhanced, pediatrics, gadolinium, gadobutrol, expert consensus
Introduction
Magnetic resonance imaging (MRI) is an important modality for diagnosing and monitoring a wide range of childhood diseases. Gadolinium-based contrast media enhance the efficacy of MRI for many applications. Until recently, evidence to direct best practice in pediatric MRI was based largely on adult studies, but pediatric-specific data are now increasingly available. However, a number of open issues remain, indicated by the large variations in practice between centers.
This review article reports current perceptions on the practice of MRI in children, based on discussions and consensus statements developed at an international expert meeting, attended by nine pediatric radiologists from internationally recognized radiology centers in Canada, Germany, Italy, South Korea, Spain, Sweden, and the UK, combined with follow-up collegiate revisions during manuscript development. The review is not intended to be comprehensive, but focuses on areas of topical interest while noting areas for further investigation. Reflecting recent clinical trial activity, the role of gadolinium-based contrast media in pediatric MRI receives particular attention.
The recommendations expressed in this review are intended solely as general guidance on best practice in pediatric MRI. Clinical decision-making must be based on the requirements of each patient, guided by the latest sources of information available, including local guidelines and newly published trial data.
Advantages of MRI in pediatric radiology
Imaging modalities available in children may be classified as invasive (eg, intra-arterial digital subtraction angiography and endoscopy) and noninvasive (eg, ultrasound, X-ray, computed tomography [CT], nuclear medicine, positron emission tomography, and MRI). In practice, all these techniques are routinely employed, since no single modality can fully replace another. The preference for particular methods depends on the local availability of each modality and on the clinical scenario, taking into account the degree of invasiveness and potential associated morbidities, including those from exposure to ionizing radiation.
CT has developed rapidly as an imaging modality. This is explained by the increasing availability of multidetector CT scanners and the ability of the technique to provide rapid, high-quality image acquisition. However, the radiation associated with CT represents a major concern, particularly for children, who are more sensitive to the effects of ionizing radiation than adults.1,2 The risk of cancer due to radiation exposure is two to three times higher in children than in adults.1,2 While specific protocols have been developed for CT with scanning parameters specifically designed for children, the best way to reduce the radiation dose to pediatric patients is to avoid unnecessary CT exams.3 Thus, alternative imaging modalities without ionizing radiation exposure, commonly ultrasound and MRI, are preferred for diagnosis in pediatric clinical practice.
Ultrasound is easy to perform and provides real-time imaging of dynamic processes at relatively low cost. In addition, there is no need for sedation. However, ultrasound does not always suffice to confirm or exclude pathology, characterize lesions, or display exact anatomic limits to plan patient management.
MRI has the capacity to provide high-resolution images of tissue anatomy in multiple planes, combined with quantitative functional imaging. A particular advantage of MRI is its ability to differentiate soft tissues. The main drawbacks of MRI relevant to pediatric imaging are the potential need for sedation or anesthesia and the limited availability of MR equipment tailored to pediatric use outside specialized centers. The long sequence time utilized in conventional MRI has the potential drawback of making timed scanning difficult, for example when both an arterial and a portal venous phase scan of the liver is required. This drawback has been minimized with newer, shorter acquisition sequences designed for contrast-enhanced MRI, such as VIBE or FLASH.
Consensus statement
MRI offers the major safety advantage of a lack of ionizing radiation, combined with efficacy benefits of excellent three-dimensional anatomic representation, tissue characterization, and quantitative/functional capabilities.
Applications of MRI in pediatric radiology
MRI is an established technique for the detection, evaluation, staging, and follow-up of a range of disease processes.4 MRI provides data on anatomy and physiologic processes (flow, diffusion, and perfusion) with high sensitivity and specificity.
The extensive experience of MRI in adult patients is often—but not always—directly transferable to the pediatric population. Pediatric MRI presents challenges that relate primarily to: (a) anatomic differences in structures, including developmental changes, (b) different physiologic parameters, (c) characteristic diseases of this age-group, and (d) behaviors typical of this age-group that limit adequate performance of an MRI study.
Specific applications of MRI in children include anatomic imaging of the central nervous system (CNS), chest, abdomen, pelvis, and musculoskeletal tissue for disorders including congenital malformations, tumors, infections, metabolic disorders, and inflammatory diseases (Table 1).4 Additional, quantitative information for the characterization of disorders can be provided by techniques including diffusion-weighted MRI, MR spectroscopy (MRS), and perfusion MRI.5–8 Diffusion-weighted MRI has particular applications to detect early cerebral ischemia and infarction, to differentiate intracranial cysts from solid masses, to diagnose encephalopathy or encephalitis, and to identify congenital anomalies; recent applications based on technological developments extend beyond the CNS to include tissue characterization (eg distinguishing benign from malignant tissue), organ function (such as for liver and kidneys), and monitoring response to therapy in extra-neurological tumors.9,10 MRS combines information from MRI with nuclear magnetic resonance to provide information on tissue metabolites that can help differentiate abnormalities such as certain types of tumors. MRS has been used to evaluate neurodegenerative diseases, including early detection and monitoring of response to therapy for demyelinating diseases (where N-acetyl aspartate [NAA] and choline levels may be increased), as well as in epilepsy and trauma (where NAA levels may be decreased); a widespread role for MRS is not yet established.11–13 Perfusion MRI, such as by arterial spin labeling (ASL), assesses relative cerebral blood flow and volume, and can be used to better characterize tumors and detect areas of ischemia during stroke.14 Increasingly, functional and quantitative techniques are being incorporated into standard MRI protocols.15
Table 1.
Applications of pediatric MRI by body region.
| BODY REGION | APPLICATIONS OF MRI | ADVANTAGES OF MRI | MR PROTOCOL |
|---|---|---|---|
| Brain and spine |
|
Extensive experience of anatomic and functional characterization of CNS pathologies | Brain: axial T2-weighted, coronal FLAIR, and coronal and sagittal T1-weighted images Spine: sagittal, fast spin-echo T1- and T2-weighted sequences Gadolinium enhancement used in suspected inflammation, tumors/metastases, white matter disorders, neurocutaneous disorders |
| Chest |
|
Superior visualization of interstitial processes, inflammatory disease | T2-weighted (turbo spin echo) T1-weighted spin echo or 3D-gradient echo sequence after applying gadolinium contrast (if suspicion of abscess, assessment of fibrosis activity) Combination of cardiac and ventilator gating often required (use fast imaging technique) |
| Cardiovascular system |
|
Provision of 3D anatomic and hemodynamic information, beyond echocardiography and catheterization | Breath-held, ECG-gated, balanced steady-state free precession (b-SSFP) cine image Gadolinium enhancement used in b-SSFP and MRA |
| Abdomen |
|
Anatomic depiction of complete abdominal organ systems Enterography (oral contrast distention of the bowel combined with intravenous gadolinium) provides increased sensitivity for bowel wall abnormalities |
Coronal T2-weighted or STIR images in combination with axial T2-weighted and/or fat suppressed (STIR) T2-weighted images Further sequences obtained according to underlying pathology Intravenous hyoscine or glucagon to reduce peristalsis Gadolinium enhancement used in suspected inflammatory bowel disease |
| Musculoskeletal system |
|
Versatile depiction of bone marrow, cartilage, joints, and soft tissues to identify and localize pathology | T1-weighted, T2-weighted, and proton density sequences (at least one combined with fat saturation), short tau inversion recovery sequence T1-weighted images performed with gadolinium contrast |
| Genitourinary tract (urography) |
|
Evolving technique for generating high-quality anatomic scans (kidneys, ureters, and bladder) and renal function assessments (eg, split renal function and drainage) | T2-weighted imaging (static-fluid MR urography) T1-weighted fat suppressed post-contrast imaging (excretory urography) |
| Infections | CNS
|
Sensitive and specific imaging, providing early diagnosis | T1- (pre- and post-gadolinium) and T2-weighted images Gadolinium enhancement provides additional information for differential diagnosis |
| Metabolic disorders and malformations |
|
Depiction of small/subtle pathology | Axial T2-weighted turbo spin echo, an axial FLAIR, T2*-weighted gradient-echo sequences, diffusion-weighted imaging and sagittal T1-weighted acquisition Gadolinium-enhanced T1-weighted images for inflammatory diseases or tumors |
| Whole body |
|
3D-anatomic visualization for determining location and extent of lesions; functional/quantitative capabilities | STIR or fat suppressed T2 spin echo, diffusion-weighted imaging, fat suppressed T1 spin echo, 3D-spoiled gradient echo sequences in arterial or portovenous phase following gadolinium contrast, fat suppressed T1 SE (post-gadolinium) |
The majority of MRI procedures in children are for CNS disorders, most frequently congenital malformations, inflammatory diseases, epilepsy, stroke, or brain tumors; the recent availability of age-specific MRI templates for neuroimaging during pediatric development provides a reference resource for normal structural changes over time.16–18 Also common are abdominal MRI to identify tumors and infections, and musculoskeletal MRI to diagnose arthritis, osteomyelitis, other bone and soft tissue infections, and tumors. MRI of the cardiovascular system is being more widely used, both alone and in combination with echocardiography, as it provides exceptional visualization of three-dimensional anatomy and reliable measures of function.19 Additional emerging applications for pediatric MRI include urography, enterography (see Fig. 7), and cine airway imaging.20 Whole body MRI, while technically demanding in children, can aid detection of disease through the entire body, with particular applications for locating multifocal lesions (eg, metastases, storage disorders, and multifocal osteomyelitis) and determining the extent of soft tissue disorders.21,22
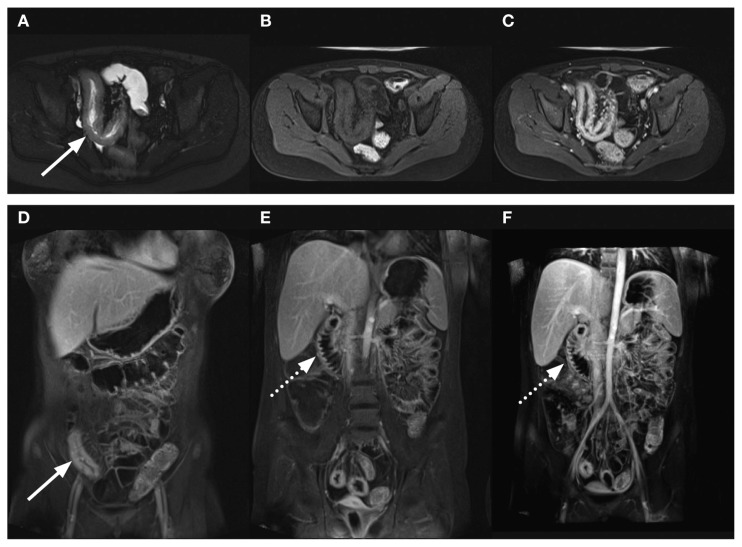
Figure 7.
Child with Crohn’s disease with inflamed terminal ileum and inflamed duodenum demonstrated by MR contrast-enhanced enterography. HASTE (A) axial images show thickening of the wall of the distal ileum (arrow) that is slightly brighter than muscle. The T1 transverse images pre- (B) and post-contrast (C) show diffuse enhancement of the thickened wall along with prominence of the vascularity in the adjacent mesentery. This is also seen in the coronal T1 contrast-enhanced images with fat saturation (arrow, D and E), along with similar abnormal enhancement of the thickened duodenal wall (dashed arrow, E and F). Courtesy Professor R Bhargava.
MRI has therefore become the modality of choice, in place of CT, in children because of the variety and types of tissue contrast it provides, combined with its non-invasiveness. Use of MRI is recommended in most clinical scenarios, particularly in follow-up to avoid repeated radiation exposure. Nonetheless, there are specific exceptions where other imaging modalities are preferred, such as the following examples:
Lung pathology: conventional X-ray and CT are preferred
Pathology of small bones (eg, temporal bone) and cortical bone lesions: CT is preferred in the emergency setting. MRI may misdiagnose lesions, but is useful for imaging complications as in acute mastoiditis23
Congenital heart disease in the newborn: CT offers greater speed in diagnosis
Multitrauma: CT offers greater speed and, usually, no requirement for sedation.
The selection of MRI over ionizing-radiation modalities is based on the availability of high-quality and high-field MR scanners, coils, and software and reflects the expertise and experience of the operator. Despite variability between centers in the current first-choice indications for imaging techniques, MRI will likely become the modality of choice for most indications in future.
Consensus statement
MRI is the modality of choice for diagnosing a broad spectrum of clinical disorders and for evaluating abnormalities detected at ultrasound or X-ray. Alternative imaging modalities currently have advantages in specific situations. In future, MRI is likely to become the first-choice modality across most indications.
Practical issues in pediatric MRI
Preparing the child
Aspects of the MRI procedure, such as the enclosed space and the loud noise from the scanner, can cause anxiety in children, especially those of younger age. An adult family member or guardian should be encouraged to stay with the child during the scan. Child life specialists are a resource available at many hospitals, offering expertise to assist pediatric patients and their parents/guardians to cope with the procedure and to provide educational information, as required.24
Sedation or anesthesia is effective for reducing anxiety and movement in approximately 90% of cases. Sedatives/hypnotics at the lowest possible dose are preferred.25 Widely used agents for sedation include:
Propofol: administered by infusion at 2–5 mg/kg/h for sedation, with advantages of short induction (2 min) and recovery (8 min) times and a low incidence of complications.26,27
Dexmedetomidine: administered as a loading dose (2–3 μg/kg, over 10 min) and maintenance infusion (1–2 μg/kg) for sedation. Dexmedetomidine is unsuitable in patients with cardiac compromise; however, less airway support may be required for dexmedetomidine than for propofol.27,28
Pentobarbital: oral or rectal dosing at 3–6 mg/kg, with a time to onset of 15–60 min and duration of effect 60–120 min.27 Pentobarbital may be associated with cardiovascular and respiratory depression.
Chloral hydrate is not recommended at many centers, based on high incidences of nausea and vomiting, long recovery time, postoperative agitation, and high failure rates for MRI.27
General anesthesia (GA) may be chosen in selected children (eg, with congenital heart defects or airway abnormalities) and particularly in patients requiring long-duration scans (eg, with staging investigations, in cases of malignancies) or with a history of failed sedation.29 In small children, the predictable safety of GA may be preferred over deep sedation; sedation also has a lower success rate.27,30
Sedation and GA carry risks of complications that necessitate continuous monitoring.25 Adverse events (AEs) of sedation, including respiratory depression and hypoxemia, may occur in up to 20% of children. Conversely, inadequate sedation, potentially leading to failure of the MRI procedure, is reported in 13% of children.31 GA can impact adversely on data acquisition, such as brain chemistry assessments in MRS.32 Sedation and GA are also costly, may be impractical, and require a recovery period. For these reasons, sedation and GA are generally avoided where possible and alternative approaches are employed. The choice of the agent and technique used for sedation or GA reflects the experience of the practitioner, potential constraints imposed by the patient and procedure, the availability of appropriate monitoring equipment (including electrocardiography, pulse oximetry, blood pressure, and body temperature assessments), and the institutional policies in place.27 All members of the anesthetic team should be familiar with MRI-specific safety issues and the requirements of the diagnostic procedure before induction.33
Familiarizing the child and parent or guardian with MRI can facilitate the MR procedure. Verbal explanation supported by explanatory literature or cartoons (for very young children) represents good practice. Novel approaches to familiarizing young patients include interactive online programs and recordings of MRI scanner noise that can be played at home.34 Exposing the patient to “mock MRI” using a scanner “shell” has been reported effective.35,36 Audio and video entertainment can be integrated into the scanner to distract the patient during the procedure.
Another approach that is especially suitable for infants is to time the scan to coincide with normal sleep patterns or following breastfeeding, or to encourage the child to remain awake until the scan, aiming for natural sleep during the examination.33 A feed-and-sleep technique with use of swaddling to reduce movement (“feed and swaddle” protocol) can successfully avoid the need for sedation in neonates and infants.37–40 Preparing the child before transfer to the scanner (eg, removing intravenous therapy equipment and monitors) can help lower anxiety. The anxiety and pain of procedure-related injections can be reduced by using anesthetic cream at the venipuncture site and, in inpatients, performing intravenous access on the ward.
Many centers offer their own recommendations on practical methods for preparing the child, including information on food intake before the scan, what to bring to the appointment, the duration of the test, and how the scan results will be communicated. Adult family members or guardians can be encouraged to become familiar with these recommendations.
Consensus statement
Staff and environment should help the patient and parent or guardian feel secure and remain calm during the MR examination. Patients can be familiarized with the procedure before the scan. Younger children may be encouraged into natural sleep during the examination. In selected cases, sedation or GA may be used, according to institutional preference.
Performing the pediatric MR examination
Scanning times
As described elsewhere, a short scan time is a desirable objective in pediatric MRI. Hardware-based strategies to minimize scan times include high field-strength magnets and multi-channel phased-array coils for enhanced image quality. Software-based strategies include fast imaging sequences (mentioned in Table 1), parallel image processing, compressed sensing, and respiratory triggering or combined respiratory-cardiac triggering methods.41,42 MR applications that utilize parallel imaging with potential to reduce scan times in wider practice include contrast-enhanced dynamic imaging, volumetric (3D) T2-weighted imaging, and single shot imaging (SSFSE, HASTE).42 Continuing advances in hardware and software are predicted to reduce scan times further in the future.
Equipment
Knowledge of the field of view of the imaging coils available in the department dictates coil choice. The size of the imaging coil should be approximately 1.5 times the size of the body region imaged. Institutions that scan children frequently may consider obtaining a selection of dedicated coils with fields of view that fit the range of anatomy to be scanned.43 Use of an array of multichannel coils permits parallel imaging, which can substantially reduce the duration of pediatric MRI, particularly of the abdomen and cardiovascular system.
The progressive introduction of 3 Tesla (T) imaging offers improved spatial resolution and signal-to-noise ratio (SNR) compared with 1.5 T.44 3 T imaging may be particularly beneficial for children because of their smaller body size, although specific coils are required (detailed in21). Low-field MRI (0.2–0.5 T) cannot be recommended in children.
Consensus statement
To achieve optimal resolution, coils should be selected according to the body region. At minimum, a 1.5 T system should be used, but a 3 T system will provide superior imaging if specific coils are available.
MRI protocol
The procedures used and their sequence in the protocol have a substantial impact on the efficacy of the MR examination. Selection of the optimal protocol for individual patients is complex, especially in children. Continued changes in technology and the relative rarity of some disorders largely preclude an evidence-based approach to protocol choice. At individual centers, factors influencing protocol choice include the equipment available, staff experience, and guidelines in place.
Fluid-attenuated inversion-recovery (FLAIR) imaging is an important component of MR examination of the brain in adults, but FLAIR sequences are not routinely recommended for patients under 1 year old, because pathology may be masked by hyperintense unmyelinated white matter. Also, GA with high content of oxygen may increase the subarachnoid signal in FLAIR imaging, which can falsely suggest bleeding.
T1- and T2-weighted sequences are recommended in all age-groups. In acute situations, in all pediatric age-groups (beginning in the newborn), diffusion-weighted imaging and gradient echo imaging are necessary for diagnosing ischemic or hemorrhagic stroke. Gradient echo imaging, by being less susceptible to motion artefacts, also has a place in bowel imaging. Time-of-flight angiographic and venographic techniques have value in assessing vascular abnormalities. Recent studies of contrast-enhanced MR angiographic (MRA) and venographic (MRV) techniques suggest benefit in the assessment of vascular pathology.45,46 MRS can be used in cases of suspected metabolic disorder and for differentiating tumor and inflammation.
Additional protocol components may include inversion recovery with inversion times set to suppress fat (STIR) in CNS, abdominal, and musculoskeletal imaging; time-resolved angiography for dynamic angiographic data (TWIST, Siemens; TRICKS, GE Healthcare; 4D-TRAK, Philips; Freeze Fame, Toshiba; and TRAQ, Hitachi); and volumetric interpolated breath-hold examination for contrast-enhanced thoracic, vascular, and abdominal imaging (VIBE, Siemens; LAVA, GE Healthcare; THRIVE, Philips; and Quick 3D, Toshiba) (see Fig. 6).
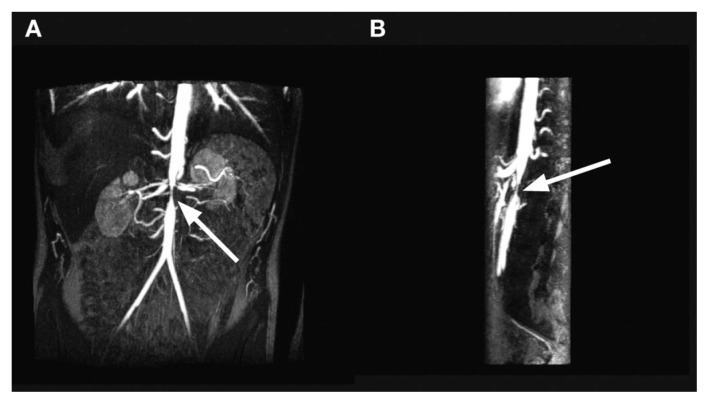
Figure 6.
15 year old with aortic and superior mesenteric artery stenosis secondary to neurofibromatosis. Maximal intensity projections of the subtracted contrast-enhanced VIBE sequences show focal narrowing of the abdominal aorta (arrow) at the level of the renal arteries in coronal (A) and sagittal (B) projections. The sagittal image also shows focal narrowing of the origin of the superior mesenteric artery, just caudal to the normal-sized celiac axis and cranial to the aortic narrowing. Courtesy Professor R Bhargava.
Readers are referred to recent reviews and recommendations for guidance on specific protocols in neurology,15 cardiology,19 respiratory medicine,47 gastroenterology,6 musculoskeletal disorders,5 and whole body imaging.48
Protocol selection—experience in clinical practice
Representative case studies of MRI procedures in children for indications including CNS, circulatory, abdominal, and soft tissue disorders are shown in Figures 1 and 2.
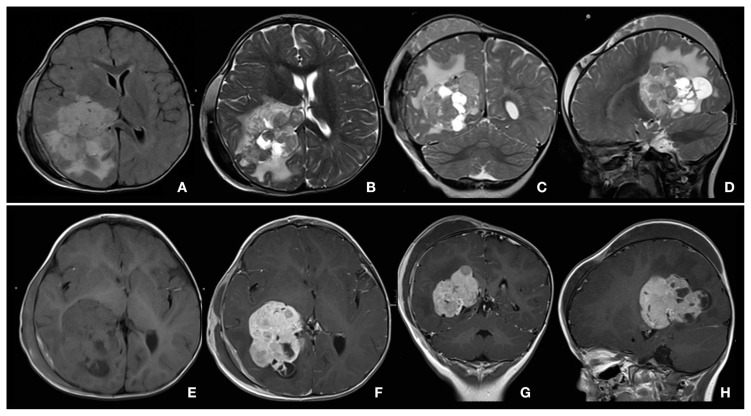
Figure 1.
Choroid plexus carcinoma of right ventricle, in 2-year-old girl with Turner syndrome, polycystic kidney, nephrolithiasis, and posttraumatic skull fracture with cephalohematoma over right hemisphere. Technique: head coil, 1.5 T, gadobutrol 1 mL by manual injection. Protocol: FLAIR, T2 TSE, T1, T1 Gd. Slice thickness 3–4 mm. Findings: Pre-contrast T2-weighted (B, C, D) and FLAIR (A) images showed a brain tumor with inhomogeneous signal in right ventricle. Surrounding parenchyma of the right hemisphere showed bright signal in T2. Post-contrast (F, G, H): inhomogeneous enhancement in the tumor with cystic changes compared with pre-contrast images (E). Conclusions: MRI provided differential diagnosis of plexus carcinoma vs. plexus papilloma. Courtesy Dr G Hahn.
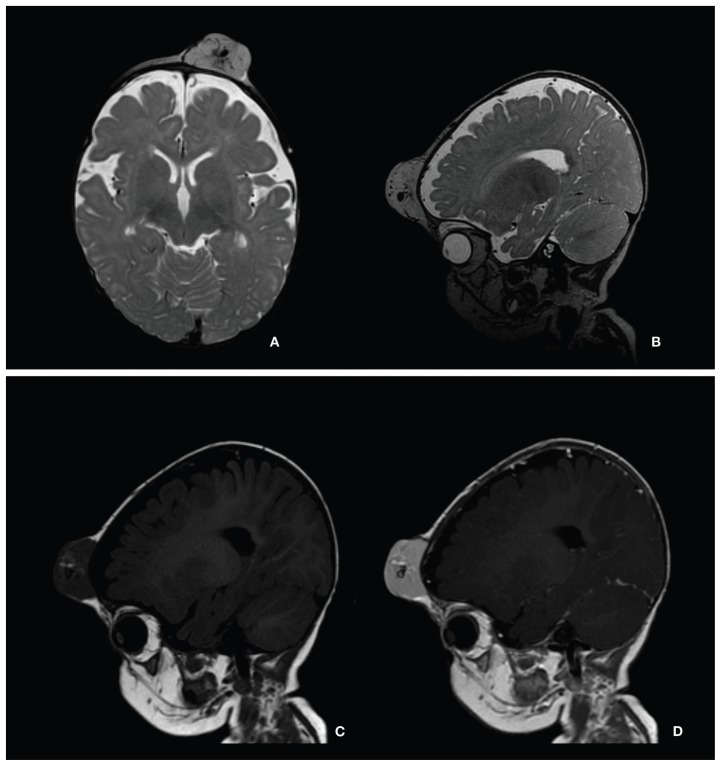
Figure 2.
Hemangioma in 7-month-old girl with large soft tissue mass in forehead. Technique: head coil, 1.5 T, gadobutrol 0.6 mL by manual injection. Protocol: T2, T1, T1 Gd. Slice thickness 1.2–3.0 mm. Findings: 3 × 4 × 2 cm tumor attached to the bony calvarium on left side on T2-weighted images (A, B). Intermediate signal on T1 (C) with small spots of higher signal and small tubular hypointensities (signal voids) within the mass. After administration of gadobutrol, the tumor enhanced uniformly, except for central vascular structures (D). No obvious intracranial extension or other pathologic findings. Conclusions: MRI with gadolinium enhancement was valuable for determining the extent of disease and associated anomalies and for excluding malformations of the brain. Courtesy Dr E Stokland.
Practical suggestions for performing MRI in children, based on expert discussions, are summarized in Table 2.
Table 2.
Practical suggestions for pediatric MRI: equipment and protocol.
| TECHNICAL RECOMMENDATIONS |
|---|
|
|
|
| PATIENT CARE RECOMMENDATIONS |
|
|
|
Abbreviations: FLAIR, fluid-attenuated inversion-recovery; GA, general anesthesia; MRI, magnetic resonance imaging; SNR, signal-to-noise ratio; STIR, short inversion-time inversion recovery; T, Tesla.
Consensus statement
MRI protocols should be selected on an individual basis, adjusting parameters appropriately to the patient’s size and condition.
Applications of contrast-enhanced MRI in pediatric radiology
Criteria for use of contrast enhancement
In many indications, gadolinium-based contrast media provide additional, clinically relevant information when compared with native MRI. Discussion of contrast enhancement in pediatric MRI can be divided into CNS (brain and spine) and non-CNS applications.
Contrast-enhanced brain and spine MRI
At many centers, gadolinium-based contrast enhancement represents the clinical standard for imaging CNS disorders, providing additional information on the location, type, and stage of lesions for diagnosis and treatment planning.49,50 Contrast-enhanced MRI improves the accuracy of differential diagnosis between CNS tumors and alternative diseases, such as demyelinating disorders (multiple sclerosis and acute disseminated encephalomyelitis) and abscesses.7 Evaluation of tumors is improved with contrast enhancement not only by looking at enhancement patterns but also for detecting metastasis indicating the malignant nature of CNS masses. Besides its role in imaging tumors, contrast-enhanced MRI is a valuable tool in characterizing CNS infections; vascular anomalies and disorders51; neurological pathologies (including demyelinating diseases and neurodegenerative disease); and neurocutaneous syndromes (such as neurofibromatosis). For bacterial infections such as meningitis and meningoencephalitis, contrast-enhanced MRI assists in monitoring the response to therapy and the development of complications such as ischemic lesions, abscess, or empyema.4,52,53 Contrast-enhanced MRI also has an important role in the diagnosis of intracranial tuberculosis and bacterial spondylodiscitis, and in detecting and monitoring viral infection and immune-mediated inflammation. Inflammatory disorders such as Guillain–Barré syndrome are better identified with contrast than on non-enhanced studies with identification of enhancing nerve roots.54,55
In addition to providing conventional images based on anatomy, MRI can characterize functional and metabolic features of cerebral tissue. Functional imaging techniques (eg, dynamic susceptibility contrast, DSC) can provide information on the relative cerebral blood volume (rCBV), which may assist in identifying the neovascularization associated with tumor growth and help to guide biopsy by localizing the most capillary-dense portion of a tumor. DSC is the current MR imaging-based technique of choice for in-vivo quantification of perfusion parameters in normal or tumor tissue.56 Following treatment, contrast-enhanced MRI can detect lesion recurrence before symptoms develop, increasing the likelihood of an improved outcome.
Contrast-enhanced non-CNS MRI
Gadolinium-based contrast-enhanced MRI is widely used for characterizing infections; inflammatory processes; neurocutaneous syndromes (eg, neurofibromatosis); abdominal, musculoskeletal, and soft tissue disorders, including tumors; cardiovascular disease and malformations; and metabolic disease. Contrast enhancement can be especially helpful for defining small or subtle lesions or foci of inflammation that are unclear on native scans.57
MR urography with contrast enhancement has become an accepted substitute for intravenous urography and scintigraphy, with the capability to combine in a single study the assessment of morphology and function, including the concentrating and excretory functions of each kidney.58 Furosemide is administered at the beginning of the study to enhance dilation of the urinary tract and aid in the distribution and dilution of gadolinium-based contrast medium. A typical protocol includes pre-contrast T1 and T2 images through the kidneys, ureters, and bladder, followed by gadolinium-based contrast medium administration for contrast enhancement and dynamic contrast-enhanced T1 imaging of the urinary tract. MR urography is particularly useful for investigation of hydronephrosis and malformations of the ureteropelvic unit.59,60
Contrast-enhanced MRA is as effective as digital subtraction angiography for the evaluation of vascular diseases.51,61 Pediatric applications of contrast-enhanced MRA include the characterization of congenital cardiovascular abnormalities of the chest, abdomen, and extremities, with superiority over cine angiography or echocardiography.62–65
Typically, local and national guidelines are in place to advise on use of contrast enhancement in different indications. While contrast enhancement offers additional information relative to unenhanced MRI in the great majority of indications, contrast media are not routinely employed for certain metabolic and musculoskeletal (eg, suspected herniated disk, bone fracture) MR imaging procedures. In children with severely impaired renal function or on dialysis, or in very young children, contrast medium use should be subjected to careful risk/benefit assessment, because of the low risk for nephrogenic systemic fibrosis (NSF, discussed below). For these groups, unenhanced MRI or other imaging techniques should be considered; for example, studies show that diffusion-weighted MRI has potential applications for the characterization of kidney function and pathology in patients with renal insufficiency.66 Guidelines should ideally allow flexibility in the use of contrast media to reflect the complexities of clinical practice. The injection method, speed, timing, and flush should all be decided on an individual patient basis.
Consensus statement
Gadolinium-based contrast media provide reliable enhancement on T1-weighted images and represent the clinical standard in many pediatric MRI protocols. Gadolinium-based contrast media improve the localization, characterization, and staging of tumors/lesions, the differentiation of inflammatory and infective disorders, and the performance of MRA.
Considerations in contrast medium choice
Readers are referred to local guidelines and prescribing information for details on the contrast media approved for use in different age-groups. Table 3 summarizes selected properties of gadolinium-containing contrast media.
Table 3.
Properties and approval status of extracellular gadolinium-based contrast agents.a
| CHEMICAL NAME | TRADE NAME | MANUFACTURER | CHARGE AND CHEMICAL STRUCTURE | CONCENTRATION (mol/L) | KINETIC STABILITYb | RELAXIVITY (3 T IN PLASMA, 37°C) [L/mmol−1s−1] | T1 SHORTENING TIME (MS) IN BLOOD FOR 1 mL/L AGENT | VISCOSITY [mPa*s] | OSMOLALITY [mOsm/kg H2O] | EXCRETION | RECOMMENDED DOSES FOR IMAGING (mmol/kg) | APPROVED DOSES FOR CHILDREN (mmol/kg) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gadodiamide | Omniscan | GE Healthcare | Nonionic linear | 0.5 | 35 s | 4.0 | 880.85 | 1.4 | 790 | Renal | Body 0.1 CNS 0.1 Kidney 0.05 Intrathoracic, intra-abdominal, pelvic 0.1 |
From 2 years: 0.1 |
| Gadopentetate dimeglumine | Magnevist | Bayer | Ionic linear | 0.5 | 10 min | 3.7 | 864.80 | 2.9 | 1960 | Renal | CNS 0.1 Extracranial/ extraspinal 0.1 Body 0.1 |
From 2 years: 0.1 |
| Gadobenate dimeglumine | MultiHance | Bracco | Ionic linear | 0.5 | N/A | 5.5 | 960.96 | 5.3 | 1970 | Renal, 4–5% hepatobiliary | CNS 0.1 MRA 0.1 |
From 2 years: 0.1 |
| Gadoversetamide | OptiMARK | Tyco | Nonionic linear | 0.5 | N/A | 4.5 | N/A | 2.0 | 1110 | Renal | CNS 0.1 Liver 0.1 |
Not approved <18 years |
| Gadoterate meglumine | Dotarem | Guerbet | Ionic cyclic | 0.5 | >1 month | 3.5 | 859.0 | 2.0 | 1350 | Renal | CNS 0.1 Extracranial/ extraspinal 0.1 Body 0.1 |
Infants and children: 0.1 |
| Gadoteridol | ProHance | Bracco | Nonionic cyclic | 0.5 | 3 h | 3.7 | 870.33 | 1.3 | 630 | Renal | CNS 0.1 Extracranial/ extraspinal 0.1 |
From 2 years: 0.1 |
| Gadobutrol | Gadovist, Gadavist | Bayer | Nonionic cyclic | 1.0 | 24 h | 5.0 | 1036.96 | 4.96 | 1390 | Renal | CNS 0.1 Liver 0.1 Kidney 0.1 MRA 0.1 Whole body (EU) 0.1 |
From 2 years: 0.1 |
| Gadoxetic acid | Primovist | Bayer | Ionic linear | 0.5 | N/A | 6.2 | N/A | 1.19 | 668 | 50% renal, 50% hepatobiliary | Liver 0.025 | Not approved <18 years |
Please consult your local prescribing information for the latest information on approved indications and dosing.
Kinetic stability: dissociation half-life at pH 1.0.
Readers are referred to recent reviews for a discussion of the potential role of organ-specific contrast media, such as gadoxetate disodium for hepatobiliary imaging, in pediatric patients.67
Clinical Trials in Pediatric MRI
Until recently, few well-controlled clinical trials were available to guide contrast medium choice in pediatric MRI, in contrast to the extensive experience in adults. The trials that were available typically included low numbers of pediatric patients in limited indications.68–71
Studies to characterize contrast agent use specifically in pediatric MRI include pharmacokinetic and safety investigations of the 0.5 molar gadolinium-based contrast media72–74 and the 1 molar agent, gadobutrol.75,76
Factors Influencing Contrast Medium Choice
Safety
Safety is the primary determinant in the choice of contrast medium. Safety considerations for each contrast medium include the stability of the molecule, AEs, and the pharmacologic profile.
Chelate stability
Gadolinium-based MRI contrast media can be classified by their molecular structure into linear and macrocyclic groups. Agents with a linear structure have a polyamino-carboxylic acid “backbone” that wraps around, but does not fully enclose, the gadolinium ion, whereas macrocyclic compounds (gadobutrol, gadoterate meglumine, and gadoteridol) possess a tetra-aza “cage” that surrounds the ion.
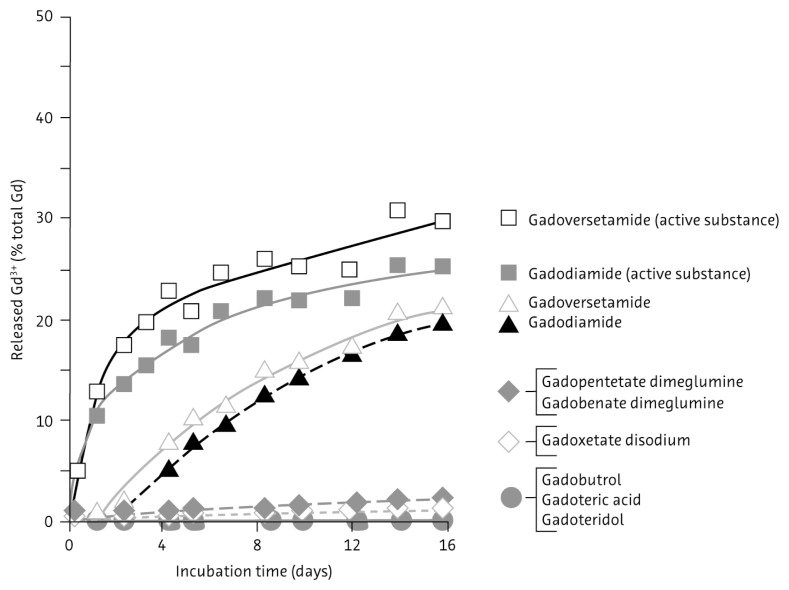
In-vitro experiments under physiologic conditions show that macrocyclic agents are more stable and less prone to release gadolinium ions than linear compounds (Fig. 3).77 Gadolinium-containing contrast media have been linked to the condition of NSF in patients with renal impairment.78,79 The stability of the chelate appears to have a role in the development of NSF.
Figure 3.
Comparative rates of gadolinium ion release for 1 molar solutions of gadolinium-based contrast media in serum from healthy volunteers at 37°C. Reproduced from Thomas Frenzel, Philipp Lengsfeld, Heiko Schirmer, Joachim Hütter, Hanns-Joachim Weinmann, Stability of Gadolinium-Based Magnetic Resonance Imaging Contrast Agents in Human Serum at 37°C, Invest Radiol, 2008;43:817–828 with permission from Wolters Kluwer Health.
Recently, the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) released guidelines on the risk of NSF associated with gadolinium-based contrast media, placing macrocyclic compounds in the low-risk category (Table 4).80 In similar initiatives, the U.S. Food and Drug Administration and the European Society of Urogenital Radiology (ESUR) also placed macrocyclic agents in the lowest-risk group for development of NSF.81,82
Table 4.
Gadolinium-based contrast media classified according to CHMP categorization of NSF risk (CHMP 2009).80
|
Abbreviations: CHMP, Committee for Medicinal Products for Human Use; NSF, nephrogenic systemic fibrosis.
Growing awareness of NSF has been accompanied by a decline in the number of reported cases. For children, in particular, incidences of NSF appear to be very rare.83,84
Adverse events/adverse drug reactions
The safety margin for diagnostic drugs should be high, particularly for those used in pediatric patients. The published literature, reflecting primarily adult MRI experience, reports that adverse reactions occur at low rates and are qualitatively similar for current gadolinium-based contrast media, regardless of molecular structure.85–88 Common adverse drug reactions (ADRs) include nausea, vomiting, and hives.
Assessments of AEs and ADRs in pediatric MRI are more problematic, reflecting the low number of age-specific studies when compared with adult MRI. In the absence of extensive clinical study data, perceptions on the safety and tolerability of contrast media in pediatric MRI may be informed by personal experience. AEs have been reported to occur at low rates in individual studies of gadodiamide, gadobutrol, gadobenate dimeglumine, gadopentetate, and gadoversetamide in pediatric patients of different ages, while a retrospective chart review reported that allergic-like reactions to gadolinium-containing contrast media were rare.72,89–92 A recent safety study of gadobutrol in 130 patients aged 2 to 17 years75 reported a tolerability profile that was comparable with adult experience,88 with low rates of AEs that were mostly mild to moderate in intensity.
Pharmacologic profile
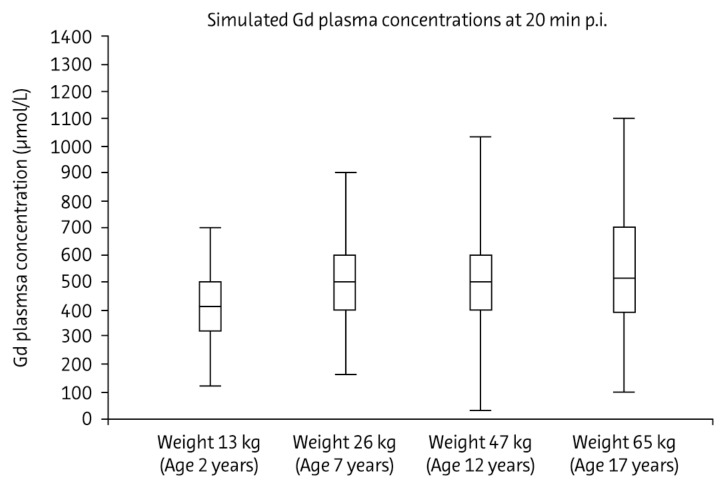
The currently available gadolinium-based contrast media display similar pharmacokinetic profiles in adults.93 Pharmacokinetic studies in children aged 2 and older have included the 0.5 molar agent, gadoversetamide,72,74 and the 1 molar agent, gadobutrol.75 These studies concluded that individual differences in pharmacokinetics (total body clearance and central volume of distribution) were attributable to body weight, with no additional effect from age (Fig. 4). Dosage based on body weight—as in adults—is therefore appropriate in children aged 2 and older, and no age-dependent dose adjustment is required. Experience of gadobutrol use in children under 2 years indicates that standard weight-adjusted dosing is feasible also with gadobutrol in this age-group.76
Figure 4.
Simulated gadolinium concentrations in plasma 20 minutes after injection of 0.1 mmol/kg body weight gadobutrol in four subjects of different ages represented by typical body weight. Boxes represent interquartile range, with the center horizontal line at median. Whiskers extend to data nearest to a distance of at most 1.5 times the interquartile range. Reproduced from Gabriele Hahn, Ina Sorge, Bernd Gruhn, Katja Glutig, Wolfgang Hirsch, Ravi Bhargava, Julia Furtner, Mark Born, Cronelia Schroder, Hakan Ahlstrom, Sylvie Kaiser, Jorg Detlev Moritz, Christian Wilhelm Kunze, Manohar Shroff, Eira Stokland, Zuzana Jirakova Trnkova, Marcus Schultze-Mosgau, Stefanie Reif, Claudia Bacher-Stier, Hans-Joachim Mentzel, Pharmacokinetics and Safety of Gadobutrol-Enhanced Magnetic Resonance Imaging in Pediatric Patients, Invest Radiol, 2009;44:776–783 with permission from Wolters Kluwer Health.
In summary, safety considerations are a priority when selecting a gadolinium-based contrast medium for contrast-enhanced MRI. From this perspective, macrocyclic contrast agents (gadobutrol, gadoterate meglumine, or gadoteridol) are preferred for pediatric use, particularly in relation to the potential risk of NSF, even if theoretical in most patients.
Efficacy
The efficacy of a contrast medium—ie, its capacity to enhance image quality—represents an important consideration. Individual contrast media have demonstrated differences in efficacy in adult studies.
The characteristics of an MRI contrast medium that determine its efficacy include its effect on shortening the T1 relaxation time. In dynamic examinations, the T1 relaxation time is also related to the gadolinium concentration of the solution. Gadolinium-containing contrast media with high T1 relaxivity (gadobenate dimeglumine and gadobutrol) demonstrate excellent image quality in adult studies.94–97 The majority of gadolinium-based contrast media are available as 0.5 molar formulations, while gadobutrol is a new-generation contrast medium available as a 1 molar formulation. An additional advantage of a higher gadolinium concentration is that a smaller injection volume may be used, which enables a more compact bolus geometry that is favorable for dynamic MRI procedures such as perfusion examinations and MRA.98–100
Data on the comparative efficacy of contrast media are available from preclinical studies and clinical studies in adults.95,99,101–108 In intraindividual trials, gadobenate dimeglumine demonstrated superior lesion enhancement and diagnostic information relative to gadopentetate or gadodiamide,106,107,109 which is explainable by the higher relaxivity of gadobenate. In similarly designed trials, gadobutrol demonstrated superior performance, including enhanced lesion detection and conspicuity, compared with the 0.5 molar agents gadopentetate and gadoterate meglumine, again attributable to the higher relaxivity of gadobutrol.96,101,110
Current guidelines indicate that efficacy results for contrast media in adult studies can be extrapolated to pediatric populations with the same indications.111 In support, a study of pediatric subjects aged 2 to 17 years confirmed the comparable efficacy of 1 molar gadobutrol in this population as in adults.75 The same may apply when comparing younger and older pediatric patients with similar disease processes.111
One study has directly compared contrast media for imaging brain and spine tumors in children, reporting significant superiority for gadobenate dimeglumine over gadopentetate in lesion visualization.112 Additional studies comparing the efficacy of contrast media in pediatric patients will aid practice in future. Experience in clinical practice supports the trial evidence of differences in efficacy between contrast media (see case study in Fig. 5).
Figure 5.
Neurofibromatosis type II diagnosed in 15-year-old girl with multiple cutaneous tumors and meningeal tumors. Technique: head coil, 1.5 T, Gd (gadopentetate dimeglumine or gadobutrol) by manual injection. Protocol: T2 TSE, T1, T1 Gd. Slice thickness 3–5 mm. Transverse (A, T1; B, T1 Gd), coronal (C, T1 Gd), and sagittal views (D, T2; E, T1; F and G, T1 Gd with gadopentetate dimeglumine and gadobutrol, respectively). Findings: Strong contrast enhancement in internal auditory canal. A high relaxivity agent (gadobutrol, 5 ml) showed strong enhancement in the cervical myelon (G vs. F). Conclusions: MRI assisted to diagnose schwannoma of the vestibular nerve at both hemispheres and also intraspinal neurofibroma. Notably, gadobutrol provided greater imaging efficacy than gadopentetate dimeglumine. Courtesy Professor H-J Mentzel.
Practical suggestions for the use of contrast media in pediatric MRI are summarized in Table 5.
Table 5.
Practical suggestions for pediatric MRI: contrast medium use.
|
Abbreviation: MRI, magnetic resonance imaging.
Consensus statements
Formulation
Gadolinium-containing contrast media are available at 0.5 molar concentrations, with the exception of the 1 molar agent, gadobutrol.
Safety
Safety is the primary consideration when selecting a contrast medium in pediatric MRI. Macrocyclic compounds (gadobutrol, gadoterate meglumine, and gadoteridol) are the most stable class of contrast media and are associated with lowest risk of NSF. Trial evidence on safety is available for a limited number of contrast media in pediatric MRI, but clinical experience indicates a similarity to adult profiles.
Efficacy
Signal enhancement in contrast-enhanced MRI is associated in adult studies to the T1 shortening effect, which is a function of relaxivity and, in dynamic scans, gadolinium concentration. Gadobutrol demonstrates superior lesion detection and conspicuity compared with 0.5 molar agents with a lower relaxivity in adult studies. The relationship between relaxivity and efficacy may also apply in pediatric imaging. Optimal SNR for dynamic MRI procedures may be provided by a high-concentration, tight bolus injection of contrast medium.
Conclusions
MRI, frequently with contrast enhancement, offers definitive diagnostic imaging, treatment guidance, and monitoring for a wide range of conditions, at low risk to the pediatric patient. Pediatric radiologists should assess the needs of patients individually, drawing on the available literature, personal experience, and the opinions of colleagues. To guide practice in the future, there is a need for more evidence-based decision making, founded on well-performed, pediatric-specific trials. The continued introduction of novel technologies and protocols, and the optimized use of contrast enhancement, are predicted to further increase applications of MRI in children.
Summary of expert meeting recommendations
Advantages of MRI in pediatric radiology
MRI has advantages over ionizing-radiation modalities in safety and efficacy for a range of indications and organ systems.
MRI provides high-resolution images of tissue anatomy in multiple planes, with the capability to perform quantitative functional imaging.
Practical issues in pediatric MRI
Preparation
Prior to the scan, the patient (with parent or guardian) should be familiarized with the examination to alleviate anxiety and reduce movement during the examination.
Younger children may be encouraged into natural sleep during the examination.
Decisions to use sedation or general anesthesia should be made on an individual patient basis, taking into account the benefits and risks.
Equipment and protocol
For optimized imaging, coil sizes should be selected according to the area of interest.
The scanner should be 1.5 T at minimum, and preferably 3 T.
Protocols should be individualized according to the patient’s age and imaging indication.
Criteria for use of contrast enhancement in pediatric MRI
Gadolinium-based contrast media: (1) aid the localization, characterization, and staging of lesions/tumors, (2) help differentiate inflammatory and infective disorders, and (3) allow MRA.
Contrast media are increasing the diagnostic value of the MR examination in many situations. In children with severely impaired renal function or on dialysis, or in very young children, contrast medium use should be subjected to careful risk/benefit assessment. For these groups, unenhanced MRI or other imaging techniques should be considered.
Considerations in choice of contrast medium
Gadolinium-containing contrast media are available at a 0.5 molar concentration, with the exception of the 1 molar agent, gadobutrol.
Safety is the primary consideration when selecting a contrast medium, preferably based on trial evidence. Macrocyclic agents (gadobutrol, gadoterate meglumine, and gadoteridol) have the highest chelate stability, associated with reduced gadolinium ion release.
Efficacy (image quality) that is confirmed in comparative trials is desirable. The signal intensity of a contrast medium is shown in adult studies to depend on its effect on T1 relaxivity.
Gadobutrol is the gadolinium-containing contrast medium with the highest relaxivity among the macrocyclic agents.
Acknowledgments
Editorial assistance in the preparation of this manuscript was provided by PAREXEL International.
Footnotes
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Author Contributions
All authors contributed to the manuscript concept, review of data, writing, and critical review of the draft. All authors approved the final version of the text.
DISCLOSURES AND ETHICS
As a requirement of publication the authors have provided signed confirmation of their compliance with ethical and legal obligations including but not limited to compliance with ICMJE authorship and competing interests guidelines, that the article is neither under consideration for publication nor published elsewhere, of their compliance with legal and ethical guidelines concerning human and animal research participants (if applicable), and that permission has been obtained for reproduction of any copyrighted material. This article was subject to blind, independent, expert peer review. The reviewers reported no competing interests.
FUNDING: The preparation of the review article and the consensus meeting on which it is based were funded by an unrestricted educational grant from Bayer HealthCare. Editorial assistance was funded by Bayer HealthCare.
REFERENCES
- 1.Brenner DJ. Estimating cancer risks from pediatric CT: going from the qualitative to the quantitative. Pediatr Radiol. 2002;32(4):228–231. doi: 10.1007/s00247-002-0671-1. [DOI] [PubMed] [Google Scholar]
- 2.Brenner DJ, Elliston CD, Hall EJ, Berdon WE. Estimates of the cancer risks from pediatric CT radiation are not merely theoretical: comment on “point/counterpoint: in x-ray computed tomography, technique factors should be selected appropriate to patient size. Against the proposition”. Med Phys. 2001;28(11):2387–2388. doi: 10.1118/1.1415074. [DOI] [PubMed] [Google Scholar]
- 3.McCollough CH, Primak AN, Braun N, Kofler J, Yu L, Christner J. Strategies for reducing radiation dose in CT. Radiol Clin North Am. 2009;47(1):27–40. doi: 10.1016/j.rcl.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.ACR practice guideline for the performance and interpretation of pediatric magnetic resonance imaging. American College of Radiology; [Accessed July 25, 2013]. http://www.acr.org/~/media/6DA1E94ED99645CDB414AB325414F542.pdf. [DOI] [PubMed] [Google Scholar]
- 5.Balassy C, Hormann M. Role of MRI in paediatric musculoskeletal conditions. Eur J Radiol. 2008;68(2):245–258. doi: 10.1016/j.ejrad.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Hormann M. MR imaging of the gastro-intestinal tract in children. Eur J Radiol. 2008;68(2):271–277. doi: 10.1016/j.ejrad.2008.07.019. [DOI] [PubMed] [Google Scholar]
- 7.Vezina LG. Imaging of central nervous system tumors in children: advances and limitations. J Child Neurol. 2008;23(10):1128–1135. doi: 10.1177/0883073808320753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callen DJ, Shroff MM, Branson HM, et al. MRI in the diagnosis of pediatric multiple sclerosis. Neurology. 2009;72(11):961–967. doi: 10.1212/01.wnl.0000338629.01627.54. [DOI] [PubMed] [Google Scholar]
- 9.Battal B, Akgun V, Kocaoglu M. Diffusion-weighted MRI beyond the central nervous system in children. Diagn Interv Radiol. 2012;18(3):288–297. doi: 10.4261/1305-3825.DIR.4923-11.1. [DOI] [PubMed] [Google Scholar]
- 10.Utsunomiya H. Diffusion MRI abnormalities in pediatric neurological disorders. Brain Dev. 2011;33(3):235–242. doi: 10.1016/j.braindev.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 11.Aaen GS, Holshouser BA, Sheridan C, et al. Magnetic resonance spectroscopy predicts outcomes for children with nonaccidental trauma. Pediatrics. 2010;125(2):295–303. doi: 10.1542/peds.2008-3312. [DOI] [PubMed] [Google Scholar]
- 12.Cecil KM. MR spectroscopy of metabolic disorders. Neuroimaging Clin N Am. 2006;16(1):87–116. viii. doi: 10.1016/j.nic.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 13.Thomas B, Al DN, Widjaja E. MRI of childhood epilepsy due to inborn errors of metabolism. AJR Am J Roentgenol. 2010;194(5):W367–W374. doi: 10.2214/AJR.09.2948. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, Licht DJ, Smith SE, et al. Arterial spin labeling perfusion MRI in pediatric arterial ischemic stroke: initial experiences. J Magn Reson Imaging. 2009;29(2):282–290. doi: 10.1002/jmri.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Triulzi F. Paediatric neuroimaging. Neurol Sci. 2008;29(Suppl 3):342–345. doi: 10.1007/s10072-008-1013-3. [DOI] [PubMed] [Google Scholar]
- 16.Saunders DE, Thompson C, Gunny R, Jones R, Cox T, Chong WK. Magnetic resonance imaging protocols for paediatric neuroradiology. Pediatr Radiol. 2007;37(8):789–797. doi: 10.1007/s00247-007-0462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanchez CE, Richards JE, Almli CR. Age-specific MRI templates for pediatric neuroimaging. Dev Neuropsychol. 2012;37(5):379–399. doi: 10.1080/87565641.2012.688900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanchez CE, Richards JE, Almli CR. Neurodevelopmental MRI brain templates for children from 2 weeks to 4 years of age. Dev Psychobiol. 2012;54(1):77–91. doi: 10.1002/dev.20579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bailliard F, Hughes ML, Taylor AM. Introduction to cardiac imaging in infants and children: techniques, potential, and role in the imaging work-up of various cardiac malformations and other pediatric heart conditions. Eur J Radiol. 2008;68(2):191–198. doi: 10.1016/j.ejrad.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 20.Shenoy-Bhangle A, Nimkin K, Gee MS. Pediatric imaging: current and emerging techniques. J Postgrad Med. 2010;56(2):98–102. doi: 10.4103/0022-3859.65273. [DOI] [PubMed] [Google Scholar]
- 21.MacKenzie JD, Vasanawala SS. Advances in pediatric MR imaging. Magn Reson Imaging Clin N Am. 2008;16(3):385–402. doi: 10.1016/j.mric.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 22.MacKenzie JD, Vasanawala SS. State-of-the-art in pediatric body and musculoskeletal magnetic resonance imaging [abstract] Semin Ultrasound CT MR. 2010;31(2):86–99. doi: 10.1053/j.sult.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Vazquez E, Castellote A, Piqueras J, et al. Imaging of complications of acute mastoiditis in children. Radiographics. 2003;23(2):359–372. doi: 10.1148/rg.232025076. [DOI] [PubMed] [Google Scholar]
- 24.McGee K. The role of a child life specialist in a pediatric radiology department. Pediatr Radiol. 2003;33(7):467–474. doi: 10.1007/s00247-003-0900-2. [DOI] [PubMed] [Google Scholar]
- 25.Coté CJ, Wilson S. Guidelines for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures: an update. Pediatrics. 2006;118(6):2587–2602. doi: 10.1542/peds.2006-2780. [DOI] [PubMed] [Google Scholar]
- 26.Machata AM, Willschke H, Kabon B, Kettner SC, Marhofer P. Propofol-based sedation regimen for infants and children undergoing ambulatory magnetic resonance imaging. Br J Anaesth. 2008;101(2):239–243. doi: 10.1093/bja/aen153. [DOI] [PubMed] [Google Scholar]
- 27.Schulte-Uentrop L, Goepfert MS. Anaesthesia or sedation for MRI in children. Curr Opin Anaesthesiol. 2010;23(4):513–517. doi: 10.1097/ACO.0b013e32833bb524. [DOI] [PubMed] [Google Scholar]
- 28.Mahmoud M, Gunter J, Donnelly LF, Wang Y, Nick TG, Sadhasivam S. A comparison of dexmedetomidine with propofol for magnetic resonance imaging sleep studies in children. Anesth Analg. 2009;109(3):745–753. doi: 10.1213/ane.0b013e3181adc506. [DOI] [PubMed] [Google Scholar]
- 29.Wachtel RE, Dexter F, Dow AJ. Growth rates in pediatric diagnostic imaging and sedation. Anesth Analg. 2009;108(5):1616–1621. doi: 10.1213/ane.0b013e3181981f96. [DOI] [PubMed] [Google Scholar]
- 30.Heng Vong C, Bajard A, Thiesse P, Bouffet E, Seban H, Marec BP. Deep sedation in pediatric imaging: efficacy and safety of intravenous chlorpromazine. Pediatr Radiol. 2012;42(5):552–561. doi: 10.1007/s00247-011-2310-1. [DOI] [PubMed] [Google Scholar]
- 31.Malviya S, Voepel-Lewis T, Eldevik OP, Rockwell DT, Wong JH, Tait AR. Sedation and general anaesthesia in children undergoing MRI and CT: adverse events and outcomes. Br J Anaesth. 2000;84(6):743–748. doi: 10.1093/oxfordjournals.bja.a013586. [DOI] [PubMed] [Google Scholar]
- 32.Macmaster FP, Rosenberg DR. Preparing children for MRI. Pediatr Radiol. 2008;38(3):270. doi: 10.1007/s00247-007-0706-8. [DOI] [PubMed] [Google Scholar]
- 33.Olsen OE. MRI: how to perform a pediatric scan. Acta Radiol. 2013 Feb 6; doi: 10.1177/0284185112474917. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Carter AJ, Greer ML, Gray SE, Ware RS. Mock MRI reducing the need for anaesthesia in children. Pediatr Radiol. 2010;40(8):1368–1374. doi: 10.1007/s00247-010-1554-5. [DOI] [PubMed] [Google Scholar]
- 35.de Amorim e Silva CJ, Mackenzie A, Hallowell LM, Stewart SE, Ditchfield MR. Practice MRI reducing the need for sedation and general anaesthesia in children undergoing MRI. Australas Radiol. 2006;50(4):319–323. doi: 10.1111/j.1440-1673.2006.01590.x. [DOI] [PubMed] [Google Scholar]
- 36.de Bie HM, Boersma M, Wattjes MP, et al. Preparing children with a mock scanner training protocol results in high quality structural and functional MRI scans. Eur J Pediatr. 2010;169(9):1079–1085. doi: 10.1007/s00431-010-1181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fogel MA, Pawlowski TW, Harris MA, et al. Comparison and usefulness of cardiac magnetic resonance versus computed tomography in infants six months of age or younger with aortic arch anomalies without deep sedation or anesthesia. Am J Cardiol. 2011;108(1):120–125. doi: 10.1016/j.amjcard.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Golan A, Marco R, Raz H, Shany E. Imaging in the newborn: infant immobilizer obviates the need for anesthesia. Isr Med Assoc J. 2011;13(11):663–665. [PubMed] [Google Scholar]
- 39.Mathur AM, Neil JJ, McKinstry RC, Inder TE. Transport, monitoring, and successful brain MR imaging in unsedated neonates. Pediatr Radiol. 2008;38(3):260–264. doi: 10.1007/s00247-007-0705-9. [DOI] [PubMed] [Google Scholar]
- 40.Windram J, Grosse-Wortmann L, Shariat M, Greer ML, Crawford MW, Yoo SJ. Cardiovascular MRI without sedation or general anesthesia using a feed-and-sleep technique in neonates and infants. Pediatr Radiol. 2012;42(2):183–187. doi: 10.1007/s00247-011-2219-8. [DOI] [PubMed] [Google Scholar]
- 41.Vasanawala SS, Alley MT, Hargreaves BA, Barth RA, Pauly JM, Lustig M. Improved pediatric MR imaging with compressed sensing. Radiology. 2010;256(2):607–616. doi: 10.1148/radiol.10091218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasanawala SS, Lustig M. Advances in pediatric body MRI. Pediatr Radiol. 2011;41(Suppl 2):549–554. doi: 10.1007/s00247-011-2103-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jaramillo D, Laor T. Pediatric musculoskeletal MRI: basic principles to optimize success. Pediatr Radiol. 2008;38(4):379–391. doi: 10.1007/s00247-007-0645-4. [DOI] [PubMed] [Google Scholar]
- 44.Dagia C, Ditchfield M. 3T MRI in paediatrics: challenges and clinical applications. Eur J Radiol. 2008;68(2):309–319. [Google Scholar]
- 45.Alfke K, Jensen U, Pool C, et al. Contrast-enhanced magnetic resonance angiography in stroke diagnostics: additional information compared with time-of-flight magnetic resonance angiography? Clin Neuroradiol. 2011;21(1):5–10. doi: 10.1007/s00062-010-0039-0. [DOI] [PubMed] [Google Scholar]
- 46.Spuentrup E, Wiethoff AJ, Parsons EC, Spangenberg P, Stracke CP. High spatial resolution magnetic resonance imaging of experimental cerebral venous thrombosis with a blood pool contrast agent. Eur J Radiol. 2010;74(3):445–452. doi: 10.1016/j.ejrad.2009.03.061. [DOI] [PubMed] [Google Scholar]
- 47.Hirsch W, Sorge I, Krohmer S, Weber D, Meier K, Till H. MRI of the lungs in children. Eur J Radiol. 2008;68(2):278–288. doi: 10.1016/j.ejrad.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 48.Olsen OE. Practical body MRI-A paediatric perspective. Eur J Radiol. 2008;68(2):299–308. doi: 10.1016/j.ejrad.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 49.Mentzel HJ, Seidel J, Fitzek C, et al. Pediatric brain MRI in neurofibromatosis type I. Eur Radiol. 2005;15(4):814–822. doi: 10.1007/s00330-004-2433-y. [DOI] [PubMed] [Google Scholar]
- 50.Runge VM, Muroff LR, Jinkins JR. Central nervous system: review of clinical use of contrast media. Top Magn Reson Imaging. 2001;12(4):231–263. doi: 10.1097/00002142-200108000-00003. [DOI] [PubMed] [Google Scholar]
- 51.Hentsch A, Aschauer MA, Balzer JO, et al. Gadobutrol-enhanced moving-table magnetic resonance angiography in patients with peripheral vascular disease: a prospective, multi-centre blinded comparison with digital subtraction angiography. Eur Radiol. 2003;13(9):2103–2114. doi: 10.1007/s00330-003-1844-5. [DOI] [PubMed] [Google Scholar]
- 52.Kastrup O, Wanke I, Maschke M. Neuroimaging of infections of the central nervous system. Semin Neurol. 2008;28(4):511–522. doi: 10.1055/s-0028-1083688. [DOI] [PubMed] [Google Scholar]
- 53.Nickerson JP, Richner B, Santy K, et al. Neuroimaging of pediatric intracranial infection—part 1: techniques and bacterial infections. J Neuroimaging. 2012;22(2):e42–e51. doi: 10.1111/j.1552-6569.2011.00700.x. [DOI] [PubMed] [Google Scholar]
- 54.Mulkey SB, Glasier CM, El-Nabbout B, et al. Nerve root enhancement on spinal MRI in pediatric Guillain-Barré syndrome. Pediatr Neurol. 2010;43(4):263–269. doi: 10.1016/j.pediatrneurol.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 55.Yikilmaz A, Doganay S, Gumus H, Per H, Kumandas S, Coskun A. Magnetic resonance imaging of childhood Guillain-Barre syndrome. Childs Nerv Syst. 2010;26(8):1103–1108. doi: 10.1007/s00381-010-1197-8. [DOI] [PubMed] [Google Scholar]
- 56.Lobel U, Sedlacik J, Reddick WE, et al. Quantitative diffusion-weighted and dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging analysis of T2 hypointense lesion components in pediatric diffuse intrinsic pontine glioma. AJNR Am J Neuroradiol. 2011;32(2):315–322. doi: 10.3174/ajnr.A2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kan J, Young R, Yu C, Hernanz-Schulman M. Clinical impact of gadolinium in the MRI diagnosis of musculoskeletal infection in children. Pediatr Radiol. 2010;40(7):1197–1205. doi: 10.1007/s00247-010-1557-2. [DOI] [PubMed] [Google Scholar]
- 58.Grattan-Smith JD, Little SB, Jones RA. MR urography in children: how we do it. Pediatr Radiol. 2008;38(Suppl 1):S3–17. doi: 10.1007/s00247-007-0618-7. [DOI] [PubMed] [Google Scholar]
- 59.Vivier PH, Dolores M, Taylor M, Elbaz F, Liard A, Dacher JN. MR urography in children. Part 1: how we do the F0 technique. Pediatr Radiol. 2010;40(5):732–738. doi: 10.1007/s00247-009-1538-5. [DOI] [PubMed] [Google Scholar]
- 60.Vivier PH, Dolores M, Taylor M, Dacher JN. MR urography in children. Part 2: how to use ImageJ MR urography processing software. Pediatr Radiol. 2010;40(5):739–746. doi: 10.1007/s00247-009-1536-7. [DOI] [PubMed] [Google Scholar]
- 61.Green D, Parker D. CTA and MRA: visualization without catheterization. Semin Ultrasound CT MR. 2003;24(4):185–191. doi: 10.1016/s0887-2171(03)90011-4. [DOI] [PubMed] [Google Scholar]
- 62.ACR-NASCI-SPR practice guideline for the performance of body magnetic resonance angiography (MRA) American College of Radiology; [Accessed July 25, 2013]. http://www.acr.org/~/media/D1BC4FB23D4B4005872FDDAE018E0CE7.pdf. [Google Scholar]
- 63.Saleh RS, Singhal A, Lohan D, Duckwiler G, Finn P, Ruehm S. Assessment of cerebral arteriovenous malformations with high temporal and spatial resolution contrast-enhanced magnetic resonance angiography: a review from protocol to clinical application. Top Magn Reson Imaging. 2008;19(5):251–257. doi: 10.1097/RMR.0b013e3181a98d5f. [DOI] [PubMed] [Google Scholar]
- 64.Valsangiacomo ER, Levasseur S, McCrindle BW, MacDonald C, Smallhorn JF, Yoo SJ. Contrast-enhanced MR angiography of pulmonary venous abnormalities in children. Pediatr Radiol. 2003;33(2):92–98. doi: 10.1007/s00247-002-0789-1. [DOI] [PubMed] [Google Scholar]
- 65.Zhong Y, Jaffe RB, Zhu M, Sun A, Li Y, Gao W. Contrast-enhanced magnetic resonance angiography of persistent fifth aortic arch in children. Pediatr Radiol. 2007;37(3):256–263. doi: 10.1007/s00247-006-0385-x. [DOI] [PubMed] [Google Scholar]
- 66.Thoeny HC, De Keyzer F. Diffusion-weighted MR imaging of native and transplanted kidneys. Radiology. 2011;259(1):25–38. doi: 10.1148/radiol.10092419. [DOI] [PubMed] [Google Scholar]
- 67.Tamrazi A, Vasanawala SS. Functional hepatobiliary MR imaging in children. Pediatr Radiol. 2011;41(10):1250–1258. doi: 10.1007/s00247-011-2086-3. [DOI] [PubMed] [Google Scholar]
- 68.Bonnerot V, Sebag G, de Montalembert M, et al. Gadolinium-DOTA enhanced MRI of painful osseous crises in children with sickle cell anemia. Pediatr Radiol. 1994;24(2):92–95. doi: 10.1007/BF02020160. [DOI] [PubMed] [Google Scholar]
- 69.Burry MV, Cohen J, Mericle RA. Use of gadolinium as an intraarterial contrast agent for pediatric neuroendovascular procedures. J Neurosurg. 2004;100(2 Suppl Pediatrics):150–155. doi: 10.3171/ped.2004.100.2.0150. [DOI] [PubMed] [Google Scholar]
- 70.Debatin JF, Nadel SN, Gray L, et al. Phase III clinical evaluation of gadoteridol injection: experience in pediatric neuro-oncologic MR imaging. Pediatr Radiol. 1992;22(2):93–98. doi: 10.1007/BF02011303. [DOI] [PubMed] [Google Scholar]
- 71.Kirchin MA, Pirovano G, Venetianer C, Spinazzi A. Safety assessment of gadobenate dimeglumine (MultiHance): extended clinical experience from phase I studies to post-marketing surveillance. J Magn Reson Imaging. 2001;14(3):281–294. doi: 10.1002/jmri.1184. [DOI] [PubMed] [Google Scholar]
- 72.Baker JF, Kratz LC, Stevens GR, Wible JH., Jr Pharmacokinetics and safety of the MRI contrast agent gadoversetamide injection (OptiMARK) in healthy pediatric subjects. Invest Radiol. 2004;39(6):334–339. doi: 10.1097/01.rli.0000124455.11402.52. [DOI] [PubMed] [Google Scholar]
- 73.Breslau J, Jarvik JG, Haynor DR, Longstreth WT, Jr, Kent DL, Maravilla KR. MR contrast media in neuroimaging: a critical review of the literature. AJNR Am J Neuroradiol. 1999;20(4):670–675. [PMC free article] [PubMed] [Google Scholar]
- 74.Wible JH, Jr, Tata PN, Napoli AM, Lowe LH, Kearns GL. Pharmacokinetics of gadoversetamide injection, a gadolinium-based contrast agent, in pediatric patients. Magn Reson Imaging. 2009;27(4):512–518. doi: 10.1016/j.mri.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 75.Hahn G, Sorge I, Gruhn B, et al. Pharmacokinetics and safety of gadobutrol-enhanced magnetic resonance imaging in pediatric patients. Invest Radiol. 2009;44(12):776–783. doi: 10.1097/RLI.0b013e3181bfe2d2. [DOI] [PubMed] [Google Scholar]
- 76.Bhargava R, Noga M. Safety and efficacy of gadobutrol-enhanced MRI in patients aged under 2 years—a single-center, observational study. Magnetic Resonance Insights. 2013;6:1–12. doi: 10.4137/MRI.S10996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frenzel T, Lengsfeld P, Schirmer H, Hutter J, Weinmann HJ. Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Invest Radiol. 2008;43(12):817–828. doi: 10.1097/RLI.0b013e3181852171. [DOI] [PubMed] [Google Scholar]
- 78.Kuo PH. Gadolinium-containing MRI contrast agents: important variations on a theme for NSF. J Am Coll Radiol. 2008;5(1):29–35. doi: 10.1016/j.jacr.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 79.Morcos SK. Extracellular gadolinium contrast agents: differences in stability. Eur J Radiol. 2008;66(2):175–179. doi: 10.1016/j.ejrad.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 80.European Medicines Agency. European Medicines Agency makes recommendations to minimise risk of nephrogenic systemic fibrosis with gadolinium-containing contrast agents. EMEA press office. [Accessed July 25, 2013]. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2009/11/WC500015569.pdf.
- 81.Thomsen HS, Morcos SK, Almen T, et al. Nephrogenic systemic fibrosis and gadolinium-based contrast media: updated ESUR Contrast Medium Safety Committee guidelines. Eur Radiol. 2013;23(2):307–318. doi: 10.1007/s00330-012-2597-9. [published online ahead of print Aug 4, 2012] [DOI] [PubMed] [Google Scholar]
- 82.U.S. Food and Drug Administration. FDA Drug Safety Communication: New warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. U.S. Department of Health and Human Services; [Accessed July 25, 2013]. http://www.fda.gov/Drugs/DrugSafety/ucm223966. [Google Scholar]
- 83.Foss C, Smith JK, Ortiz L, Hanevold C, Davis L. Gadolinium-associated nephrogenic systemic fibrosis in a 9-year-old boy. Pediatr Dermatol. 2009;26(5):579–582. doi: 10.1111/j.1525-1470.2008.00802.x. [DOI] [PubMed] [Google Scholar]
- 84.Perez-Rodriguez J, Lai S, Ehst BD, Fine DM, Bluemke DA. Nephrogenic systemic fibrosis: incidence, associations, and effect of risk factor assessment—report of 33 cases. Radiology. 2009;250(2):371–377. doi: 10.1148/radiol.2502080498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bleicher AG, Kanal E. Assessment of adverse reaction rates to a newly approved MRI contrast agent: review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol. 2008;191(6):W307–W311. doi: 10.2214/AJR.07.3951. [DOI] [PubMed] [Google Scholar]
- 86.Herborn CU, Honold E, Wolf M, et al. Clinical safety and diagnostic value of the gadolinium chelate gadoterate meglumine (Gd-DOTA) Invest Radiol. 2007;42(1):58–62. doi: 10.1097/01.rli.0000248893.01067.e5. [DOI] [PubMed] [Google Scholar]
- 87.Nelson KL, Gifford LM, Lauber-Huber C, Gross CA, Lasser TA. Clinical safety of gadopentetate dimeglumine. Radiology. 1995;196(2):439–443. doi: 10.1148/radiology.196.2.7617858. [DOI] [PubMed] [Google Scholar]
- 88.Voth M, Rosenberg M, Breuer J. Safety of gadobutrol, a new generation of contrast agents: experience from clinical trials and postmarketing surveillance. Invest Radiol. 2011;46(11):663–671. doi: 10.1097/RLI.0b013e3182218dc3. [DOI] [PubMed] [Google Scholar]
- 89.Dillman JR, Ellis JH, Cohan RH, Strouse PJ, Jan SC. Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am J Roentgenol. 2007;189(6):1533–1538. doi: 10.2214/AJR.07.2554. [DOI] [PubMed] [Google Scholar]
- 90.Hanquinet S, Christophe C, Greef DD, Gordon P, Perlmutter N. Clinical evaluation of gadodiamide injection in paediatric MR imaging. Pediatr Radiol. 1996;26(11):806–810. doi: 10.1007/BF01396206. [DOI] [PubMed] [Google Scholar]
- 91.Lowe LH, Kearns GL, Wible JH., Jr The safety and efficacy of neuroimaging with gadoversetamide injection in pediatric patients. Curr Med Res Opin. 2006;22(12):2515–2524. doi: 10.1185/030079906X159452. [DOI] [PubMed] [Google Scholar]
- 92.Lundby B, Gordon P, Hugo F. MRI in children given gadodiamide injection: safety and efficacy in CNS and body indications. Eur J Radiol. 1996;23(3):190–196. doi: 10.1016/s0720-048x(96)01088-1. [DOI] [PubMed] [Google Scholar]
- 93.Brown JJ, Hynes MR, Wible JH., Jr Measurement of serum calcium concentration after administration of four gadolinium-based contrast agents to human volunteers. AJR Am J Roentgenol. 2007;189(6):1539–1544. doi: 10.2214/AJR.07.2464. [DOI] [PubMed] [Google Scholar]
- 94.Anzalone N, Scarabino T, Venturi C, et al. Cerebral neoplastic enhancing lesions: multicenter, randomized, crossover intraindividual comparison between gadobutrol (1.0M) and gadoterate meglumine (0.5M) at 0.1 mmol Gd/kg body weight in a clinical setting. Eur J Radiol. 2013;82(1):139–145. doi: 10.1016/j.ejrad.2011.07.005. [published online ahead of print Sept 3, 2011] [DOI] [PubMed] [Google Scholar]
- 95.Essig M, Lodemann KP, Le Huu M, Bruning R, Kirchin M, Reith W. Intraindividual comparison of gadobenate dimeglumine and gadobutrol for cerebral magnetic resonance perfusion imaging at 1.5 T. Invest Radiol. 2006;41(3):256–263. doi: 10.1097/01.rli.0000191333.19068.6b. [DOI] [PubMed] [Google Scholar]
- 96.Kim ES, Chang JH, Choi HS, Kim J, Lee SK. Diagnostic yield of double-dose gadobutrol in the detection of brain metastasis: intraindividual comparison with double-dose gadopentetate dimeglumine. AJNR Am J Neuroradiol. 2010;31(6):1055–1058. doi: 10.3174/ajnr.A2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tombach B, Benner T, Reimer P, et al. Do highly concentrated gadolinium chelates improve MR brain perfusion imaging? Intraindividually controlled randomized crossover concentration comparison study of 0.5 versus 1.0 mol/L gadobutrol. Radiology. 2003;226(3):880–888. doi: 10.1148/radiol.2263011970. [DOI] [PubMed] [Google Scholar]
- 98.Fenchel M, Franow A, Martirosian P, et al. 1 M Gd-chelate (gadobutrol) for multislice first-pass magnetic resonance myocardial perfusion imaging. Br J Radiol. 2007;80(959):884–892. doi: 10.1259/bjr/34610669. [DOI] [PubMed] [Google Scholar]
- 99.Hadizadeh DR, Von Falkenhausen M, Kukuk GM, et al. Contrast material for abdominal dynamic contrast-enhanced 3D MR angiography with parallel imaging: intraindividual equimolar comparison of a macrocyclic 1.0 M gadolinium chelate and a linear ionic 0.5 M gadolinium chelate. AJR Am J Roentgenol. 2010;194(3):821–829. doi: 10.2214/AJR.09.3306. [DOI] [PubMed] [Google Scholar]
- 100.Willinek WA, Hadizadeh DR, von Falkenhausen M, et al. 4D time-resolved MR angiography with keyhole (4D-TRAK): more than 60 times accelerated MRA using a combination of CENTRA, keyhole, and SENSE at 3.0 T. J Magn Reson Imaging. 2008;27(6):1455–1460. doi: 10.1002/jmri.21354. [DOI] [PubMed] [Google Scholar]
- 101.Anzalone N, Gerevini S, Scotti R, Vezzulli P, Picozzi P. Detection of cerebral metastases on magnetic resonance imaging: intraindividual comparison of gadobutrol with gadopentetate dimeglumine. Acta Radiol. 2009;50(8):933–940. doi: 10.1080/02841850903095385. [DOI] [PubMed] [Google Scholar]
- 102.Anzalone N. Comparative studies of different gadolinium agents in brain tumors: differences between gadolinium chelates and their possible influence on imaging features. AJNR Am J Neuroradiol. 2010;31(6):981–982. doi: 10.3174/ajnr.A2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Attenberger UI, Runge VM, Jackson CB, et al. Comparative evaluation of lesion enhancement using 1 M gadobutrol vs. 2 conventional gadolinium chelates, all at a dose of 0.1 mmol/kg, in a rat brain tumor model at 3 T. Invest Radiol. 2009;44(5):251–256. doi: 10.1097/RLI.0b013e31819ba711. [DOI] [PubMed] [Google Scholar]
- 104.Attenberger UI, Runge VM, Morelli JN, Williams J, Jackson CB, Michaely HJ. Evaluation of gadobutrol, a macrocyclic, nonionic gadolinium chelate in a brain glioma model: Comparison with gadoterate meglumine and gadopentetate dimeglumine at 1.5 T, combined with an assessment of field strength dependence, specifically 1.5 versus 3 T. J Magn Reson Imaging. 2010;31(3):549–555. doi: 10.1002/jmri.22089. [DOI] [PubMed] [Google Scholar]
- 105.Giesel FL, Mehndiratta A, Risse F, et al. Intraindividual comparison between gadopentetate dimeglumine and gadobutrol for magnetic resonance perfusion in normal brain and intracranial tumors at 3 Tesla. Acta Radiol. 2009;50(5):521–530. doi: 10.1080/02841850902787685. [DOI] [PubMed] [Google Scholar]
- 106.Maravilla KR, Maldjian JA, Schmalfuss IM, et al. Contrast enhancement of central nervous system lesions: multicenter intraindividual crossover comparative study of two MR contrast agents. Radiology. 2006;240(2):389–400. doi: 10.1148/radiol.2402051266. [DOI] [PubMed] [Google Scholar]
- 107.Rowley HA, Scialfa G, Gao PY, et al. Contrast-enhanced MR imaging of brain lesions: a large-scale intraindividual crossover comparison of gadobenate dimeglumine versus gadodiamide. AJNR Am J Neuroradiol. 2008;29(9):1684–1691. doi: 10.3174/ajnr.A1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tombach B, Bohndorf K, Brodtrager W, et al. Comparison of 1.0 M gadobutrol and 0.5 M gadopentate dimeglumine-enhanced MRI in 471 patients with known or suspected renal lesions: results of a multicenter, single-blind, interindividual, randomized clinical phase III trial. Eur Radiol. 2008;18(11):2610–2619. doi: 10.1007/s00330-008-1054-2. [DOI] [PubMed] [Google Scholar]
- 109.Kuhn MJ, Picozzi P, Maldjian JA, et al. Evaluation of intraaxial enhancing brain tumors on magnetic resonance imaging: intraindividual crossover comparison of gadobenate dimeglumine and gadopentetate dimeglumine for visualization and assessment, and implications for surgical intervention. J Neurosurg. 2007;106(4):557–566. doi: 10.3171/jns.2007.106.4.557. [DOI] [PubMed] [Google Scholar]
- 110.Anzalone N, Colosimo C. Cerebral neoplastic enhancing lesions: Intra-individual comparison of gadoterate (Dotarem 0.5 M) and gadobutrol (Gadovist 1.0 M) at 0.1 mmol Gd/kg body weight and 1.5 T [abstract]. ECR 2010; March 4–8; Vienna, Austria. 2010. [Google Scholar]
- 111.International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Working Group. ICH Harmonised Tripartite Guideline. Clinical Investigation of Medicinal Products in the Pediatric Population E11, Current Step 4 version. International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) website. [Accessed July 25, 2013]. http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E11/Step4/E11_Guideline.pdf.
- 112.Colosimo C, Demaerel P, Tortori-Donati P, et al. Comparison of gadobenate dimeglumine (Gd-BOPTA) with gadopentetate dimeglumine (Gd-DTPA) for enhanced MR imaging of brain and spine tumours in children. Pediatr Radiol. 2005;35(5):501–510. doi: 10.1007/s00247-004-1392-4. [DOI] [PubMed] [Google Scholar]