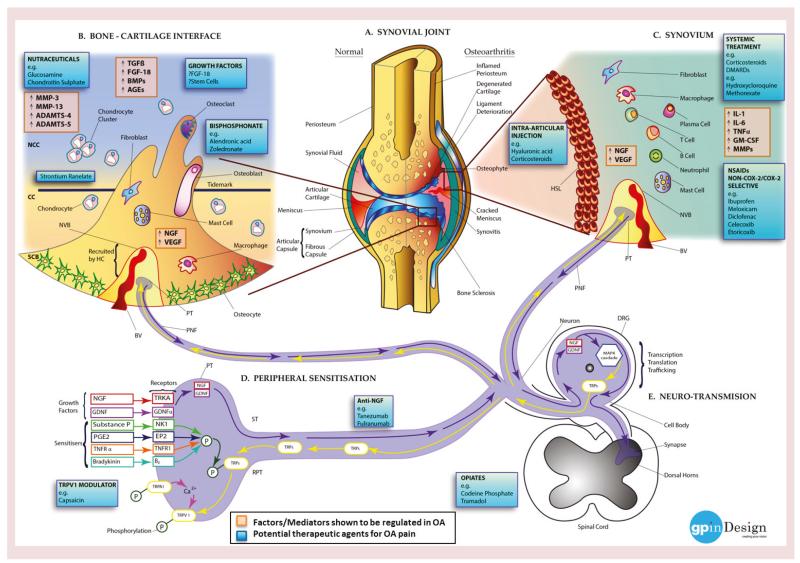
Figure 3. Molecular mechanisms of pain in osteoarthritis (see facing page).
(A) Left: normal joint structure; right: changes taking place in the synovial joint during the development of OA. (B) Bone cells (osteoblasts. osteoclasts and osteocytes), fibroblasts, macrophages and mast cells from the SCB invade the CC and the NCC, via microfractures in the bone–cartilage interface. The disruption of the osteochondral junction promotes production of enzymes, growth factors and molecules (ADAMTS-4, ADAMTS-5, AGEs, BMPs, FGF-18, MMP-3, MMP-13, NGF, TGF-β and VEGF) by the invading cells and chondrocytes stimulating innervation and vascularization ultimately recruiting NVBs (BV and PNF) from the HC. (C) Inflammation in the synovium or synovitis causes joint swelling and effusion. Hyperplasia is seen in the synovial tissue resulting in the formation of a HSL followed by the infiltration of inflammatory cells – possibly owing to a systemic response or secondary to cartilage degradation or bone marrow lesion formation. Factors, enzymes and cytokines (GM-CSF, IL-1 family, IL-6 family, MMPs, NGF, TNF-α, and VEGF) stimulated via the cells encourage innervation and angiogenesis (NVB: BV and PNF). (D) Peripheral inflammation, cartilage degradation and bone marrow lesions produce numerous inflammatory mediators (e.g., sensitisers: bradykinin, a peptide which causes blood vessels to dilate; E2 stimulates osteoblasts to release factors that stimulate bone resorption by osteoclasts; PGE2; substance P, a neuropeptide belonging to tachykinin neuropeptide family; and TNFR-α) and growth factors (e.g., GDNF and NGF) activating their subsequent receptors (bradykinin receptor B2, EP2, GDNF-α, NK1, TNFR1 and TRKA). The sensitizers work by phosphorylating TRPs: TRPA1 and TRPV1, facilitating the trafficking of the channels to the membrane of the PT. Once in the membrane of the PT, TRPA1 modulates calcium exchange with TRPV1 enhancing the nociceptive activity of both channels. The growth factors are transported down the neuron towards the DRG. Altered sensitization of such signaling pathways decreases the pain threshold in OA patients (RPT). (E) During ST, growth factors (GDNF and NGF) are transmitted into the cell body of the DRG, where they facilitate intracellular signaling pathways, for example, the MAPK cascade upregulating the expression of TRP channels (TRPA1/TRPV1), which are then transported via the neuron into the PNF and PT. Changes in this pathway during OA can switch the activity of the neurons to an altered state encouraging peripheral sensitization and RPT at the impaired site. Signaling pathways activated in the DRG then take effect in central processes.
AGE: Advanced glycation end product; BMP: Bone morphogenetic protein; BV: Blood vessel; CC: Calcified cartilage; DRG: Dorsal root ganglion; EP2: Prostaglandin E2 receptor; GM-CSF: Granulocyte macrophage colony-stimulating factor; HC: Haversian canal; HSL: hyperplastic synovial lining; MMP: Matrix metalloproteinase; NCC: Noncalcified cartilage; NVB: Neurovascular bundle; OA: Osteoarthritis; P: Phosphorylation; PGE2: Prostaglandin E2; PNF: Perivascular nerve fiber; PT: Peripheral terminal; RPT: Reducing the pain threshold; SCB: Subchondral bone; ST: Signal transduction; TRP: Transient receptor potential cation channel.
Image courtesy of Gayanthi Perera.