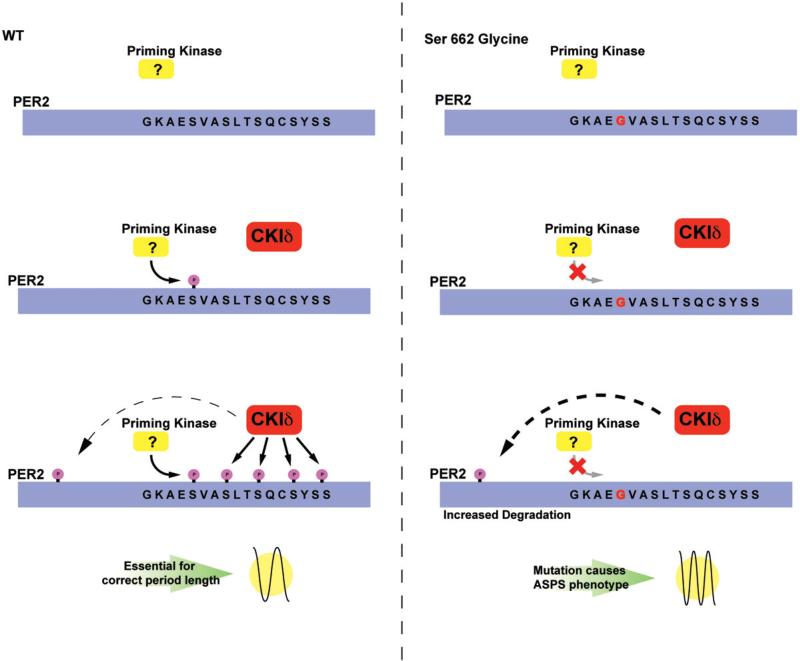
Figure 5.
Mechanism of PER2 FASP. Under normal conditions, PER2 is phosphorylated by a specific kinase at the serine 662. This initial phosphorylation “primes” the CKI domain for the subsequent recognition by CKIδ and the phosphorylation of the remaining serine residues S665–S674. The phosphorylation of these residues renders PER2 a weaker repressor and confers a longer period length. Additional phosphorylation site(s) on PER2 by CKIδ increases PER2 degradation and leads to a shorter period. Conversely, in the mutant BAC transgenic animal carrying S662G, the priming kinase cannot recognize or phosphorylate the S662 residue, leaving the protein in the hypophosphorylated state; the lack of priming prevents CKIδ from recognizing S665–S674. However, phosphorylation by CKI on the other site(s) of PER2 is unperturbed and degradation of PER2 continues without counter balance from the S662–S674 region. This then causes a shortened period and advancement of sleep phase. (see colour version of this figure at www.informahealthcare.com/bmg).