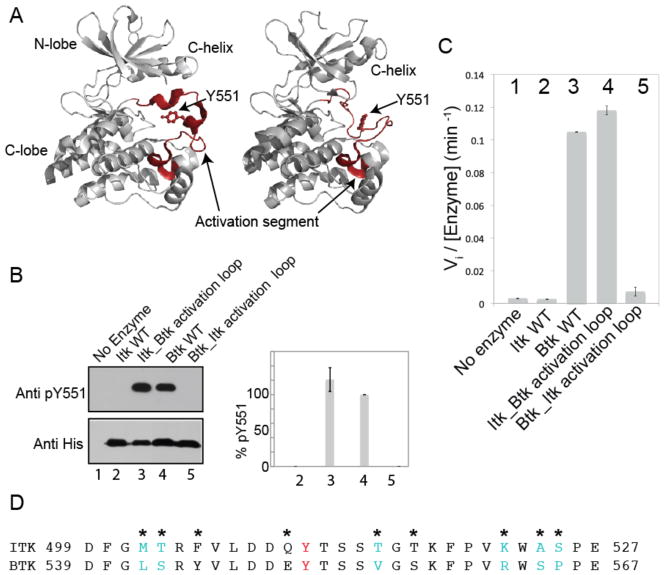
Fig. 1. Swapping the activation segments of Itk and Btk switches the catalytic activity profiles of these two related kinases.
(A) Structures of the kinase domain of Btk with the activation segment in multiple conformations [Protein Data Bank (PDB): 3GEN, 1K2P]. The N-terminal and C-terminal lobes of the kinase domain, the C-helix, and Tyr551 (Y551) on the activation loop are labeled, and the activation segment in each structure is highlighted in red and labeled. (B and C) The in vitro kinase activities of the indicated proteins were monitored by (B) Western blotting analysis for autophosphorylation on the activation loop at Tyr551 (Y551) or (C) phosphorylation of a peptide substrate (Peptide B) with 32P-ATP and determination of initial velocity (Vi). Pooled Western blotting data were quantified by normalizing the intensity of the band corresponding to pY551 of wild-type (WT) Btk (lane 4) to 100% and reporting the extent of phosphorylation of Y551 for the other proteins accordingly. Lane 1: negative control (no enzyme); lane 2: WT Itk; lane 3: the Itk_Btk activation loop; lane 4: WT Btk; lane 5: the Btk_Itk activation loop. Data in (B) and (C) are mean values ± SD from three independent experiments. The Western blot in (B) is representative of three independent experiments. (D) Amino acid sequences of the activation segments of Itk and Btk. The phosphorylation site, Y511 in Itk and Y551 in Btk is shown in red, and asterisks indicate sequence differences between the two kinases. Residues in cyan indicate the minimal sequence changes required to swap the activities of Itk and Btk [determined in (Fig. 2, A and B)].