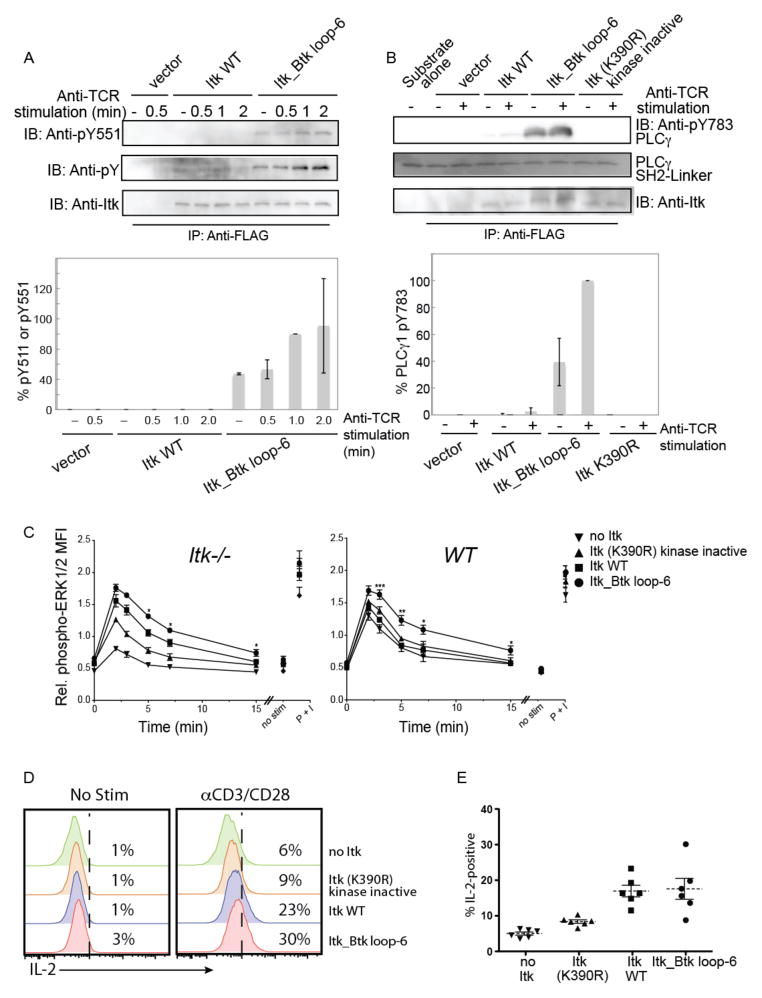
Fig. 4. Hyperactive Itk enhances phosphorylation of the activation loop and PLC-γ1 in Jurkat cells, and enhances and prolongs TCR signaling.
(A) Jurkat cells were transfected with plasmids encoding FLAG-tagged WT Itk or the Itk_Btk loop-6 mutant. Twenty four hours after transfection, cells were left unstimulated or were stimulated with C305 (anti-TCR) antibody for the indicated times. Cells were lysed, subjected to immunoprecipitation with anti-FLAG antibody, and the extent of phosphorylation on the activation loop was determined by Western blotting analysis of samples for pY511 (top blots). Data are representative of two independent experiments. Band intensities from both experiments were quantified by densitometric analysis. The abundance of pY511 in the Itk_Btk loop-6 mutant after 1 min of TCR stimulation was normalized to 100%, and the relative amounts of pY511 for the other proteins at all time points were determined. (B) WT Itk or the Itk_Btk loop mutant were immunoprecipitated from unstimulated or TCR-stimulated Jurkat cells and their activities were monitored by an in vitro kinase assay with the SH2-linker region of PLC-γ1 as a substrate. The abundance of PLC-γ1 pY783 was determined by Western blotting analysis with a specific antibody. Data are representative of two independent experiments. Band intensities from both experiments were quantified by densitometric analysis. The abundance of PLC-γ1 pY783 in the reaction containing the Itk_Btk loop-6 mutant (normalized for total Itk) after TCR stimulation was normalized to 100%, and the relative amounts of PLC-γ1 pY783 from the reactions with the other proteins were determined. (C) Itk−/− or WT OT-II TCR transgenic CD4+ T cells were infected with control retrovirus (no Itk) or with retroviruses expressing the indicated Itk constructs. Thy1.1+ cells were isolated and were left untreated or were incubated with anti-CD3 antibody (αCD3) to stimulate the TCR for 2, 3, 5, 7, or 15 min. Cells were fixed and permeabilized, and incubated with antibodies specific for CD4, Thy1.1, and pERK1/2. Graphs show the normalized mean fluorescence intensities (MFIs) ± SEM for pERK1/2 in gated CD4+ Thy1.1+ cells (see fig. S2B). Values were normalized within each experiment to the average MFI of pERK1/2 staining for all samples in that experiment, and data are means ± SEM from 4 to 6 independent experiments. Statistically significant differences between cells expressing hyperactive Itk_Btk loop-6 compared to cells expressing WT Itk are indicated: *P < 0.05; **P < 0.01; ***P < 0.001. Non-stimulated cells (no stim) were not incubated with αCD3. As a positive control for the activation of ERK1/2, cells were stimulated with PMA and ionomycin (P+I) for 4 min. (D and E) The Itk_Btk loop-6 construct does not promote increased IL-2 production after TCR stimulation of primary T cells. Itk−/− OT-II TCR transgenic CD4+ T cells were infected with control retrovirus (no Itk) or with retroviruses expressing the indicated Itk proteins. Infected cells were left untreated or were stimulated with plate-bound anti-CD3 and anti-CD28 antibodies (αCD3/CD28) for 5 to 6 hours before being incubated with fluorescently tagged antibodies specific for CD4, TCRβ, Thy1.1, and IL-2. (D) Histograms show representative flow cytometry plots of IL-2 staining in gated CD4+, TCRβ+, Thy1.1+ cells. (E) The graph shows a compilation of flow cytometry data from six independent experiments showing the percentages of cells under each condition that were positive for IL-2. Dashed horizontal lines indicate the means and bars represent the SEM. The percentages of cells expressing WT Itk or the Itk_Btk loop-6 mutant that produced IL-2 were significantly greater than those of cells expressing kinase-deficient Itk or those that had no Itk (P < 0.05). There was no statistically significant difference in the amounts of IL-2 produced by cells expressing WT Itk and those expressing they hyperactive Itk_Btk loop-6 mutant.