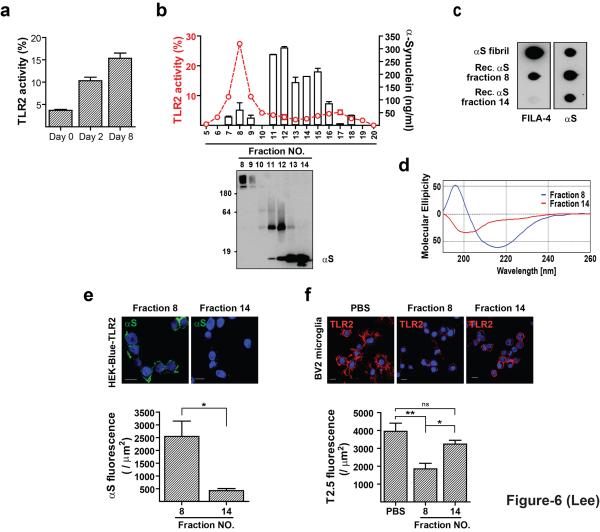
Figure 6.
Conformation-sensitive agonist activity of α-synuclein for TLR2. (a) Development of TLR2 agonist activity with endotoxin-free recombinant α-synuclein by in vitro incubation (n = 3). (b) Separation of “aged” recombinant α-synuclein by SEC. The line trace and the bars indicate TLR2 activity and the α-synuclein levels, respectively. (c) Dot blot analysis of fractions from b. (d) CD spectra of fractions 8 and 14 from b. (e) Binding of pure α-synuclein oligomers (fraction 8) on the surface of HEK293 cells expressing human TLR2/CD14 (n = 3). (f) Competition between TLR2 antibody (T2.5) and pure preparation of oligomeric α-synuclein for the binding to the surface of BV-2 microglia (n = 3). Binding data of α-synuclein oligomers (e) were analyzed by unpaired t-test. TLR2 fluorescence data (f) were analyzed using one-way ANOVA. Error bars represent ± s.e.m. *P < 0.05; **P < 0.01. “n” represents the number of independent experiments (see the methods section).