Fig. 6.
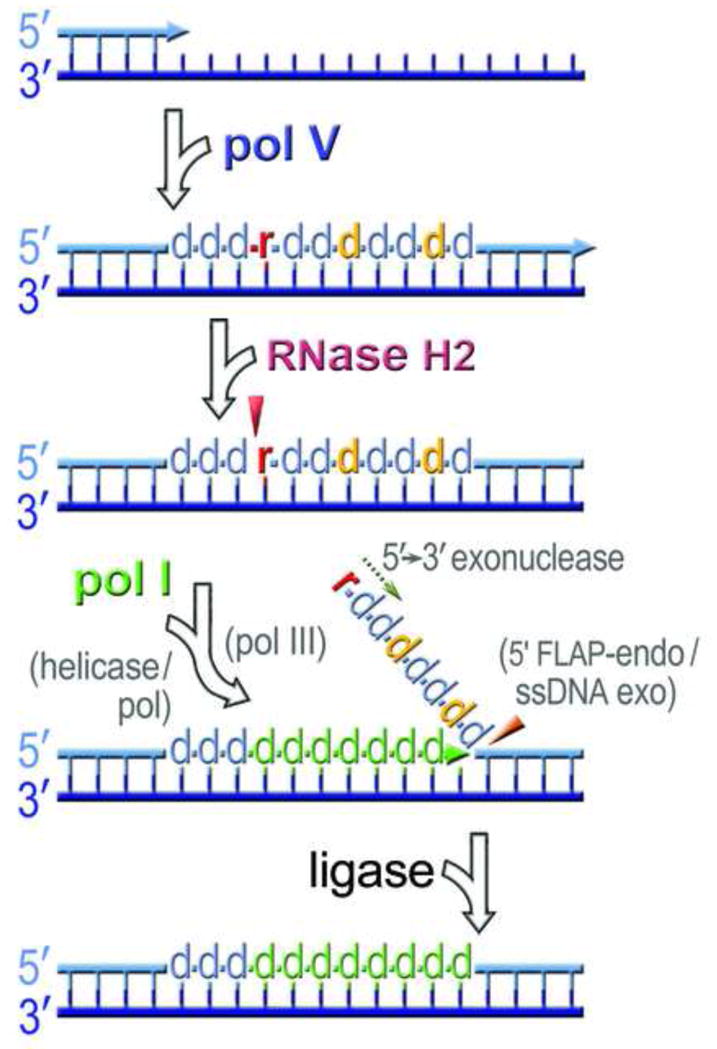
A model for ribonucleotide excision repair in E. coli. Pol V introduces multiple base substitutions (shown as d in orange) when it gains access to undamaged chromosomal DNA. It can also incorporate ribonucleotides (shown as r in red) into the nascent DNA strand. RNase HII incises the bond at the rNMP/dNMP junction 5′ to the ribonucleotide, generating DNA containing a single stranded break with 5′-phospho-ribonucleotide and 3′-hydroxyl ends. Pol I commences DNA synthesis at the nick and promotes strand displacement of the mutagenic pol V tract (shown as d in green). In polA mutants that are unable to promote strand displacement, the unwinding of the nicked intermediate proceeds through the action of an unknown DNA helicase, or polymerase (shown in parentheses). In polA_ΔC strains deficient for DNA synthesis, the 3′-hydroxyl end of the nicked intermediate is extended by high-fidelity pol III (shown in parentheses). The displaced region containing rNMP and misincorporated dNMPs is usually degraded by the 5′→3′ exonuclease or 5′-FLAP endonuclease activity of pol I, but in its absence, the displaced strand may be degraded by the FLAP endonuclease Xni, or another (yet to be identified) single-stranded nuclease (shown in parentheses). Complete repair occurs once the nick is sealed by DNA ligase. Although this model is based upon our present studies with the error-prone umuC_Y11A steric gate mutant of pol V, we envisage that identical processes occur when high-fidelity DNA polymerases inadvertently incorporate ribonucleotides into genomic DNA.
