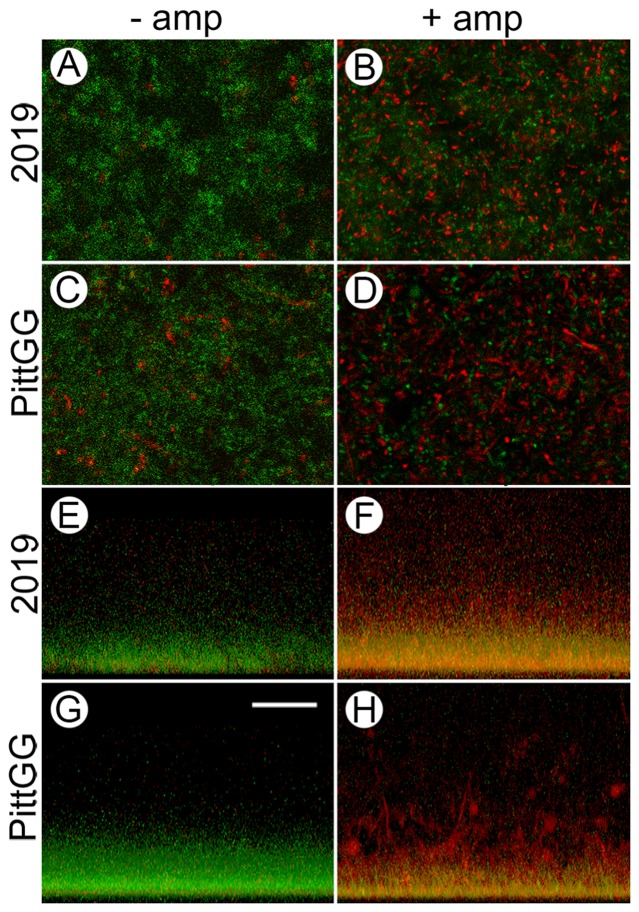
Figure 5. Sub-inhibitory concentrations of ampicillin result in an increase in dead NTHi bacteria in newly formed biofilms.
Confocal laser scanning microscopy (cLSM) of biofilms formed by NTHi strains 2019 and PittGG in the absence (− amp) and presence (+ amp) of sub-inhibitory concentrations of ampicillin were stained using the LIVE/DEAD viability assay. 2019 were exposed to 90 ng/mL ampicillin and PittGG to 170 ng/mL ampicillin. Live bacteria were colored green and dead bacteria red. From above, biofilms formed in the absence of ampicillin were mostly green (A & C), indicating living (or intact) NTHi bacteria. Clumps of bacteria in both biofilms stained red (A & C) indicating the presence of dead, or structurally compromised bacteria. Biofilms formed by 2019 (A) contained fewer aggregates of red bacteria than did PittGG (C) contained more. In the presence of ampicillin (B & D) the bacteria in the biofilms were mostly red, indicating a large number of dead bacteria. Green-stained bacteria were still present in both biofilms but appeared more aggregated in the presence of antibiotic (B & D). Large amounts of aggregated red bacteria were present in both biofilms, with the aggregates being larger in the PittGG biofilm (Fig. D). Z-stack projections of the biofilms (E–H) showed that all the biofilms were denser at the base of the biofilm, whether exposed to ampicillin or not. In the absence of ampicillin, 2019 (E) and PittGG (G) biofilms comprised green, or intact bacteria. In the presence of ampicillin the biofilm contained mostly structurally compromised bacterial cells, which were colored red or yellow. The 2019 (F) and PittGG (H) biofilms formed in the presence of ampicillin were higher than the comparable biofilms formed without exposure to ampicillin (E & G). All images; scale bar (G) = 20 µm.