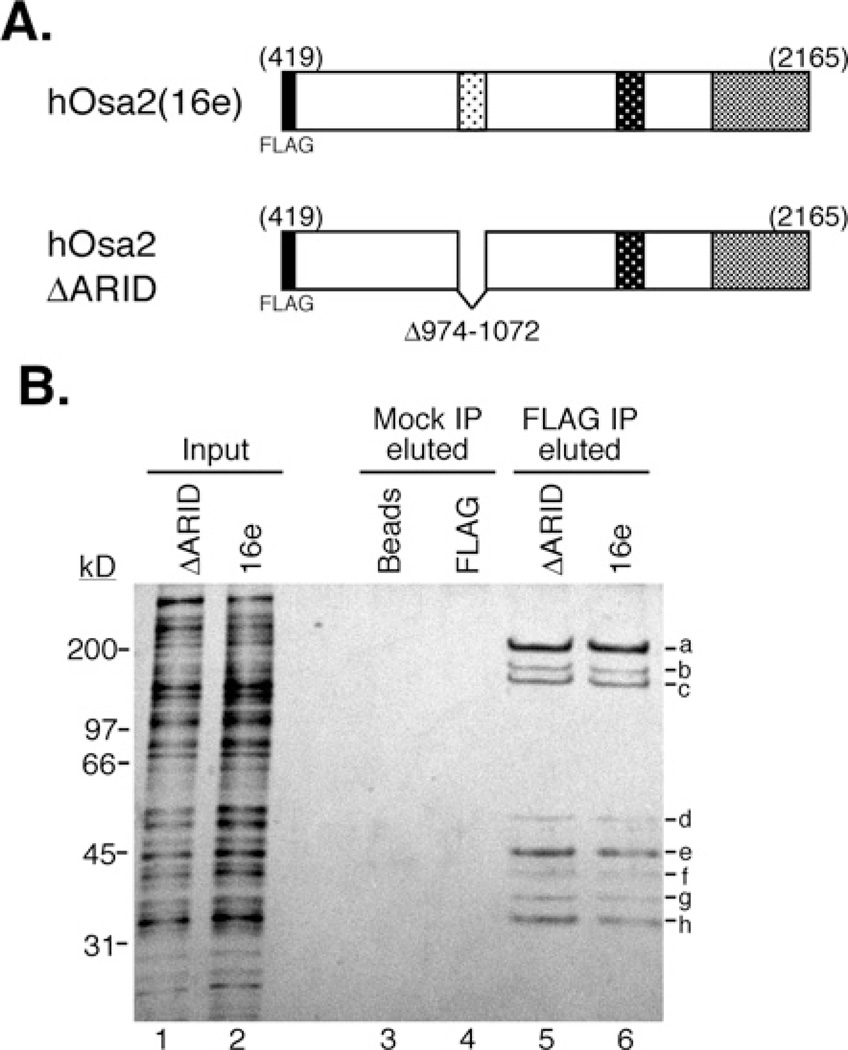
Figure 1. Purification of protein complexes associated with hOsa2 and hOsa2ΔARID.
(A) The domain structure of FLAG-tagged hOsa2 (16e) and derivative hOsa2ΔARID lacking the DNA-binding domain ARID. The three shaded regions are conserved between human and Drosophila Osa protein as described previously [13]. (B) HeLa Tet-off cells induced to express FLAG–hOsa2 (cell line 16eC3) or FLAG-hOsa2ΔARID were used to purify hOsa2-associated proteins. Nuclear extracts were loaded on to a glycerol gradient and fractions containing hOsa2 (lower third of the gradient) were pooled and subjected to immunopurification with anti-FLAG antibody and elution with FLAG peptide. The input represents 0.25% of the pooled gradient fractions (lanes 1 and 2). Mock IP (immunoprecipitation) was performed with Protein G beads (lanes 3 and 4) and eluted using the same conditions as with the anti-FLAG antibody (lanes 5 and 6). Protein bands labelled a-h were excised and subjected to analysis by mass spectrometry. Peptides for the following SWI/SNF subunits were identified: hOsa2 (a), BRG1 (a), BAF170 (b), BAF155 (b and c) and BAF60 (e). The recovery of peptides from bands d and f–h was insufficient for positive identification. The molecular mass in kDa is indicated on the left-hand side.