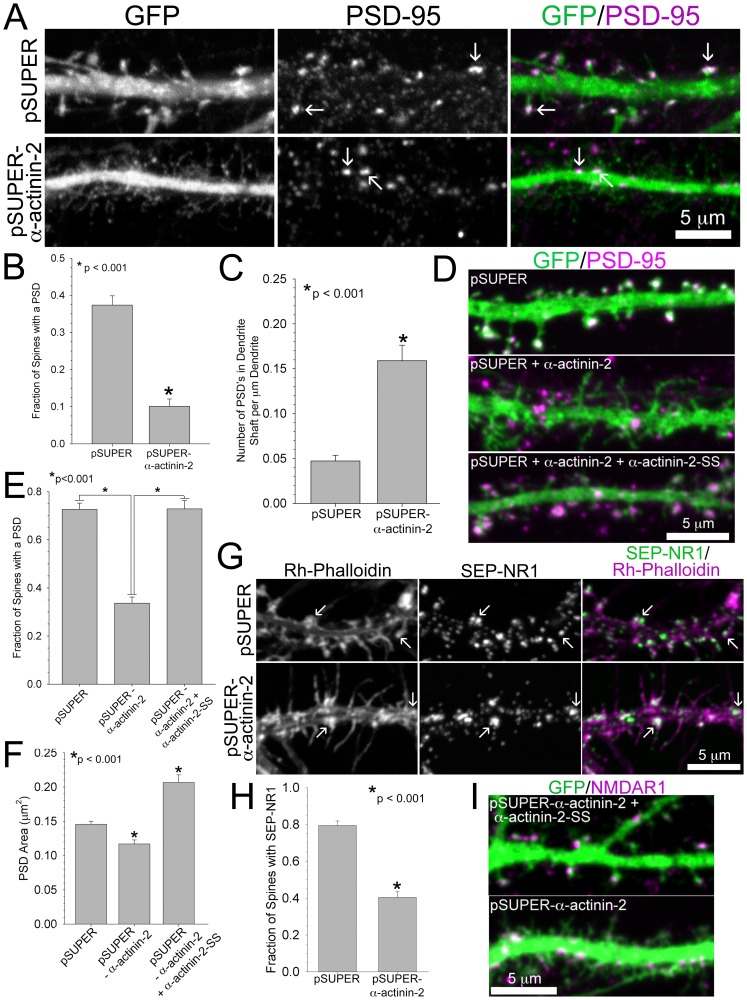
Figure 5. Loss of α-actinin-2 prevents assembly of the post-synaptic density.
A–C) α-Actinin-2 knockdown inhibits PSD assembly in spines. Hippocampal neurons were co-transfected at DIV 6 with GFP and either pSUPER or pSUPER-α-actinin-2, fixed on DIV 21, and immunostained for PSD-95. Arrows mark localization of PSD-95 in spines or in the dendrite shaft, A. The fraction of spines with localized PSD-95 is reduced in neurons with α-actinin-2 knocked down, B. PSD-95 localizes to the dendrite shaft with increased frequency in neurons lacking α-actinin-2, C. For each condition, 37-45 neurons from 5 separate cultures were analyzed. D–F) Hippocampal neurons were co-transfected at DIV 17 with GFP and either pSUPER, pSUPER-α-actinin-2, or pSUPER-α-actinin-2 plus α-actinin-2-SS, D. The fraction of spines with PSD-95 is rescued in neurons expressing exogenous α-actinin-2-SS, E. The area of PSD-95 is significantly reduced in spines lacking α-actinin-2 and rescued in neurons expressing exogenous α-actinin-2-SS, F. For each condition, PSD-95 area was measured from 543–991 spines of 23–27 neurons from 3 separate cultures. G–I) α-Actinin-2 knockdown prevents the recruitment of the NMDA receptor to the spine. Hippocampal neurons were co-transfected at DIV 6 with SEP-NR1 and either pSUPER or pSUPER-α-actinin-2, fixed on DIV 22, and immunostained for GFP and rhodamine-phalloidin. Arrows mark SEP-NR1 localization in either spines or in the dendrite, G. The fraction of spines co-localized with SEP-NR1 is reduced in neurons with α-actinin-2 knocked down, H. For each condition, 12–19 neurons from 3 separate cultures were analyzed. I) Hippocampal neurons were co-transfected at DIV 17 with GFP and either pSUPER-α-actinin-2 or pSUPER-α-actinin-2 plus α-actinin-2-SS, fixed on DIV 21, and immunostained for NMDAR1. Error bars represent SEM. p-values were derived using the paired t-test.