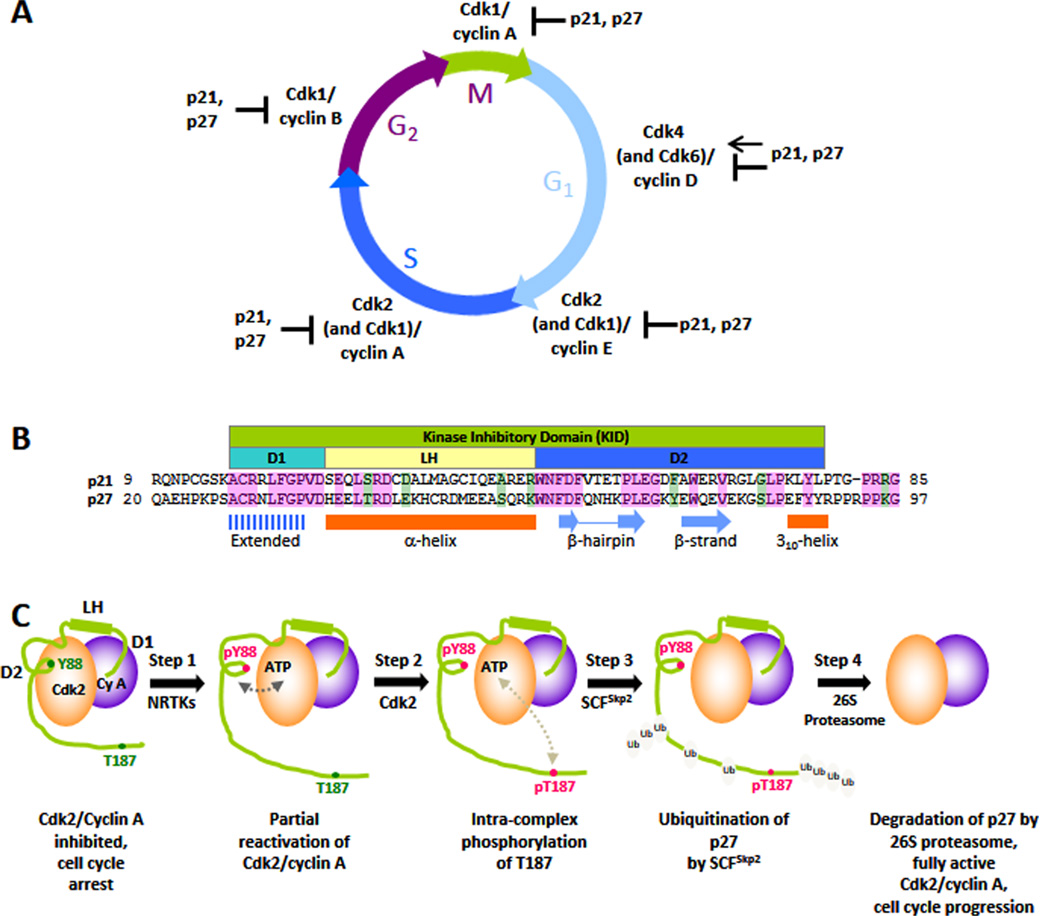
Figure 6.
Local functional unfolding promoted by phosphorylation. The disordered protein, p27, is a regulator of eukaryotic cell division (A) and folds upon binding to Cdk/cyclin complexes. The kinase inhibitory domain (B) mediates binding to Cdk/cyclin complexes, while the C-terminal domain (containing T187) remains disordered (C) and plays a critical role in phosphorylation signaling that controls the transition from G1 to S phase of the division cycle. Phosphorylation of Y88 causes unfolding of a structural element that otherwise blocks the binding of ATP to the Cdk2 active site. This so-called “regulated unfolding” reactivates Cdk2, enabling intracomplex phosphorylation of T187 and subsequent ubiquitination and degradation of p27, which fully activates Cdk2 and drives cell cycle progression. Adapted with permission from ref 376. Copyright 2012 Biochemical Society.