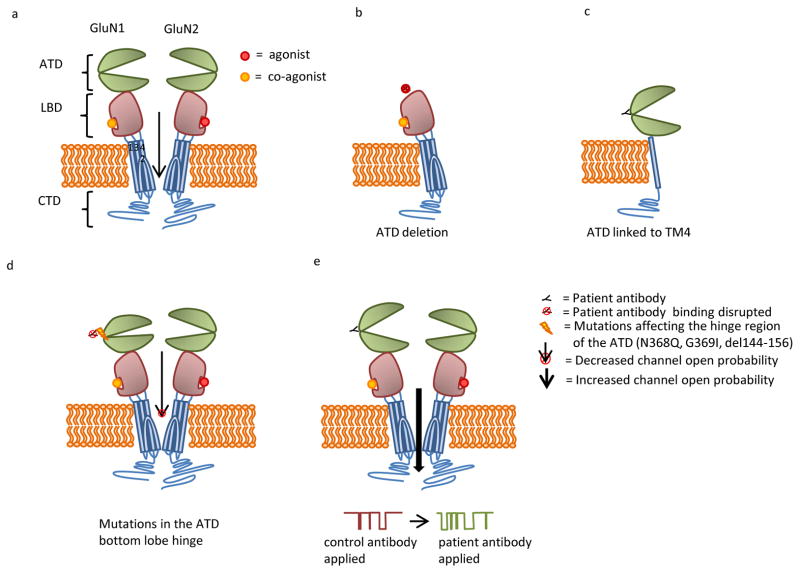
Figure 2.
(A) NMDAR subunits contain two extracellular domains: amino terminal domain (ATD) and a ligand binding domain (LBD) comprised of an S1 and S2 domain, three transmembrane domains (TMs 1, 3, 4), a transmembrane loop (TM 2), and a cytosolic c-terminal domain (CTD). GluN1 and GluN2 subunits typically combine to form a heterotetrameric receptor. (B) Deletion of the ATD eliminates patients’ antibody binding. (C) Patients’ antibodies bind to a construct that contains the ATD linked to TM4. This construct lacks the LBD and TM domains 1–3. (D) Patients’ antibody binding is eliminated by mutations to the ATD hinge region, including N368Q, G369I, and deletion of amino acids 144–156. These mutations appear to decrease channel open time. (E) Patients’ antibody binding results in increased channel open time.