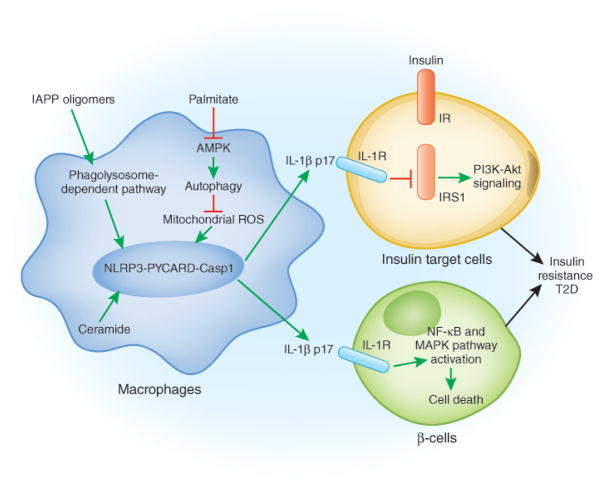
Fig 1.
A model for the pathogenesis of T2D: adipose tissue, the pancreas, NLRP3 and T2D. In adipose tissue, elevated levels of saturated free fatty acids decrease the activity of AMPK, a central regulator of energy biosynthesis and lipid metabolism, leading to defective autophagy of mitochondria (Mitophagy). The accumulation of dysfunctional mitochondria then enhances mitochondrial ROS generation and the release of mitochondrial DNA into cytosol, both of which promotes NLRP3 inflammasome activation and IL-1β release. The active IL-1β induces the activation of JNK and IKK through IL-1 receptor, which impair the insulin-insulin receptor (IR), insulin receptor substrate-1 (IRS-1), and PI3K-Akt signaling pathway. In the pancreas, the accumulation of IAPP activates the NLRP3 inflammasome and promotes IL-1β release from macrophages, which causes β-cell dysfunction and death.