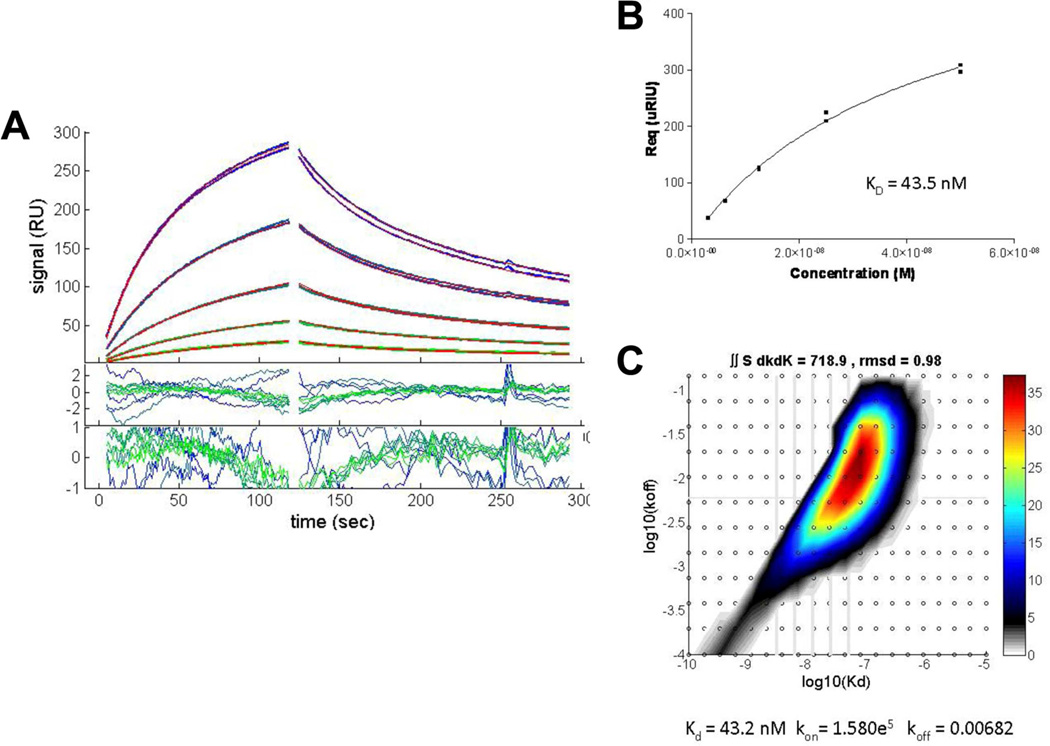
Fig 9.
SPR analysis of b12 3–5 αIM binding to the b12 Fab. (A) Association and dissociation phases of Fab binding to immobilized biotinylated b12 3–5 αIM captured on a streptavidin surface. Concentrations of Fab used were (top to bottom) 50 nM, 25 nM, 12.5 nM, 6.25 nM and 3.125 nM. (B) Langmuir isotherm of Fab binding. The Kd was determined by calculating the value of equilibrium Rmax for each injection, plotted versus concentration and fit to a Langmuir binding isotherm (Response = Equilibrium Response * ((KA*[ b12 Fab])/(KA*[ b12 Fab]+1)). (C) Distribution analysis of b12 Fab binding kinetics to surface-immobilized b12 3-5 αIM. Integration of the distribution inside a polygon drawn around the high affinity peak gives a binding capacity of 718.9 RU, an average koff = 0.0068/s, and an average KD = 43 nM.