Figure 5.
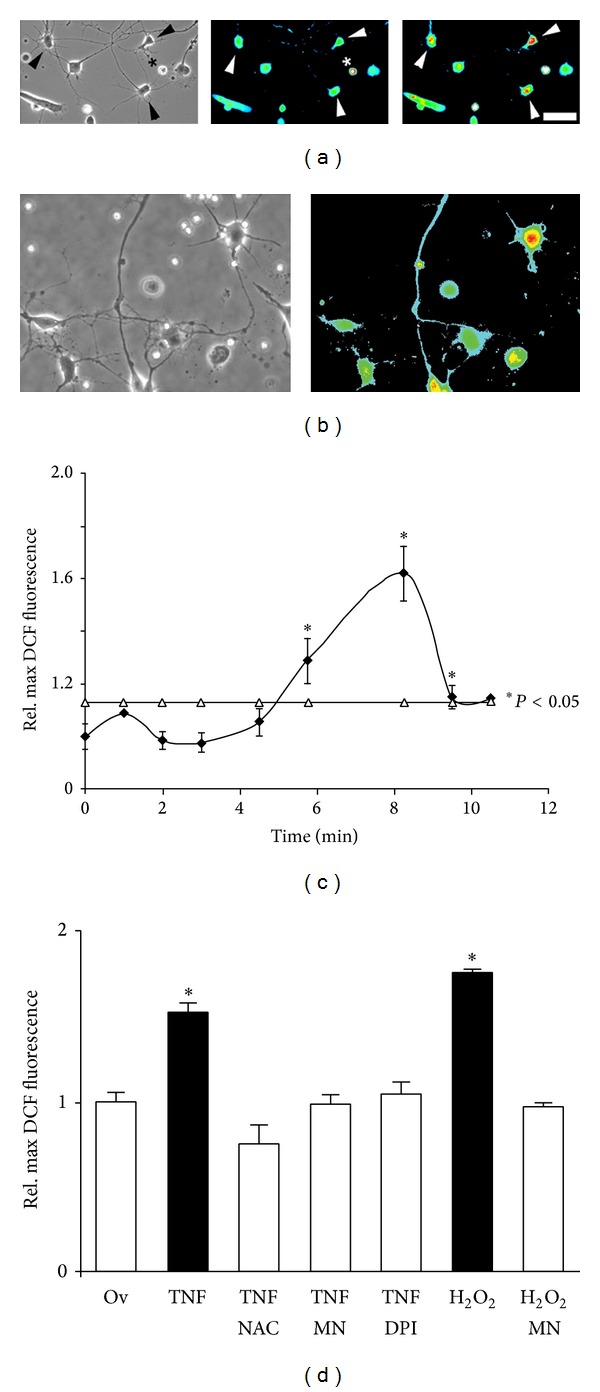
Cytokines elicit ROS formation in cell bodies of SC neurons. Dissociated E7 SC neurons were loaded with 10 μM DCF (30 min), allowed to recover for 15 min, and then incubated (45 min) with NAC, MnTBAP, DPI, or PBS (equal volume) prior to cytokines exposure. Images were acquired at identical parameters (20x). (a) DCF-loaded SC neurons exhibited a distinct neuronal morphology (arrowheads) extending one long and several shorter neuronal processes (left panel, phase contrast image). Note cultures are virtually free of nonneuronal cells. Under control conditions, SC neuron cell bodies displayed basal levels of ROS formation (middle panel, arrowheads). Degenerating cells (star) exhibit very high fluorescence intensity in the absence of any stimuli. Addition of 100 ng/mL TNFα stimulated a robust increase in intracellular ROS (right panel, arrowheads, 8 min after addition). Fluorescence images were false colored (heat spectrum) with fluorescence intensities increasing from blue (basal ROS levels) to red (high ROS levels). (b) TNFα-coated polystyrene beads increased DCF fluorescence intensity in SC neurons indicative of ROS formation. Although qualitative in nature, SC neurons with multiple bead contacts (arrow head) as opposed to single bead contact (asterisk) displayed more intense DCF fluorescence. (c) Maximum DCF fluorescence intensities per neuronal cell body were determined on a pixel-by-pixel basis after background subtraction over time and all values normalized to the average maximum DCF fluorescence intensity under control conditions at t = 0 min (relative maximum DCF fluorescence intensity). SC neurons responded with a transient increase in ROS formation in cell bodies (16 ± 2 cells per time interval) upon exposure to 100 ng/mL TNFα (2.5) min (threshold of statistical significance *P < 0.01 shown as open triangles). (d) 100 ng/mL TNFα stimulated a significant increase in ROS formation (*P < 0.01), which was negated by the presence of ROS scavengers (TNFα-NAC 2 mM or TNFα-MnTBAP 10 μM, resp.) or NOX inhibitor (TNFα-DPI 5 μM) to levels indistinguishable from 10 μg/mL ovalbumin (Ov), our control. As a control for DCF loading, 200 μM hydrogen peroxide (H2O2) greatly increased relative maximum DCF fluorescence (*P < 0.01), which was suppressed in the presence of 10 μM MnTBAP (H2O2-Mn). All measurements were obtained from at least three independent dissections (duplicate cultures each).
