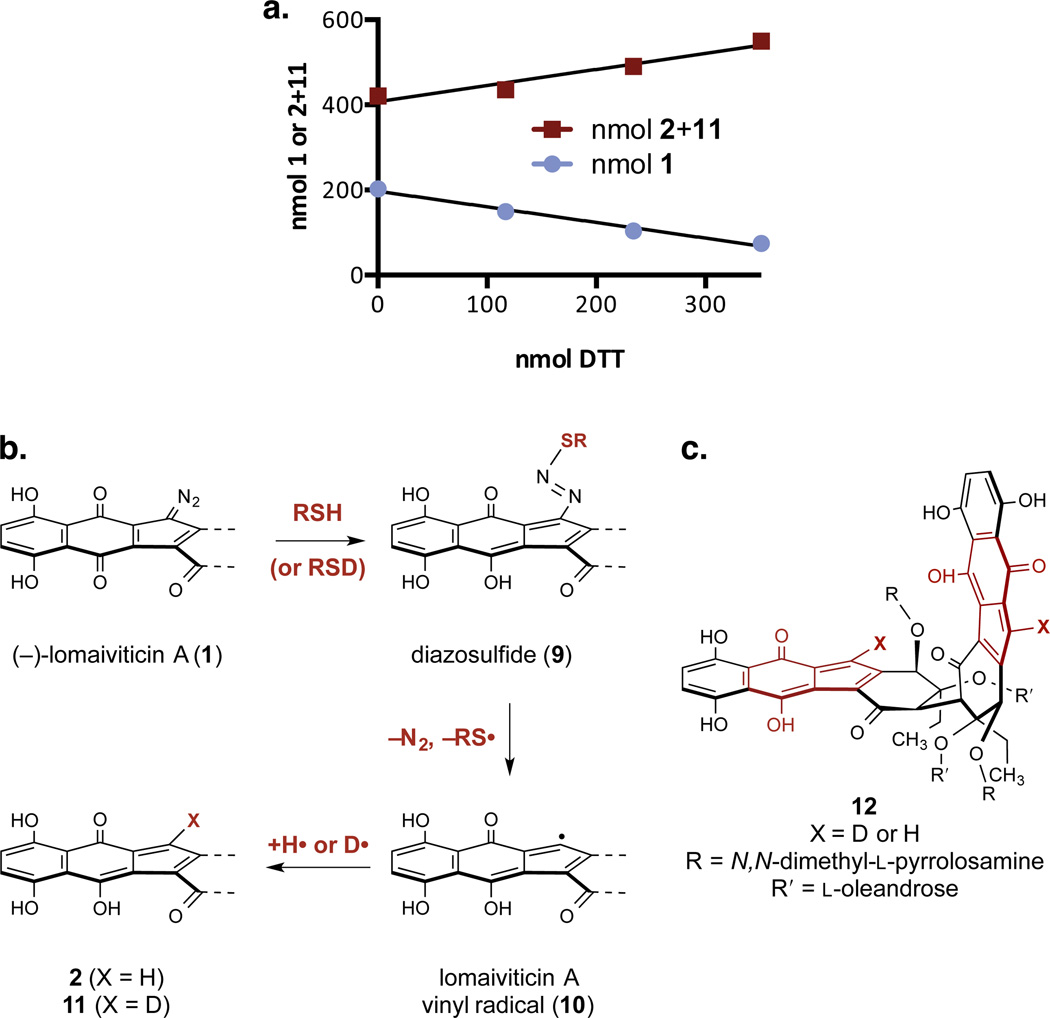
Figure 6.
Relative reactivity studies and mechanistic pathways for reduction of the diazofluorene. a. Competition hydrodediazotization experiment between (–)-lomaiviticin A (1) and (–)-lomaiviticin C (2). 1/m = –0.01163, 0.01132 for 1, 2, respectively. Conditions: 1 (202 nmol), 2 (421 nmol), DTT (3 × 117 nmol), methanol-d4 (400 µL), 21 °C. b. Postulated pathway for the transformation of 1 to 2 and 11. c. Structure of the double hydrodediazotization product 12.