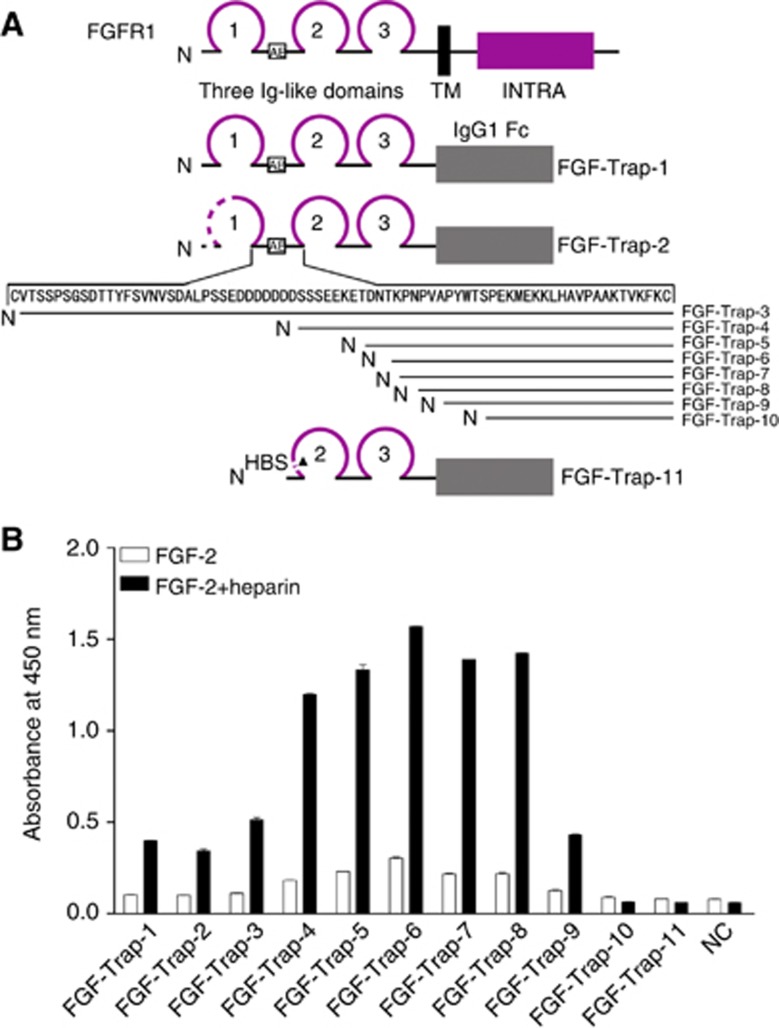
Figure 1.
Engineering and binding activity of decoy receptor fusion proteins. A) Engineering of decoy receptor fusion proteins. Multiple extracellular domains of FGFR1 were fused with the Fc portion of human IgG1. Amino terminus of various proteins is shown as N, whereas the transmembrane domain (TM), intracellular domain (INTRA) and IgG1 Fc are indicated by solid rectangles. Ig-like domains are shown as loops; 1–3 indicates the number of respective Ig-like domains. The amino-acid residues of the D1–D2 linker are indicated by arrows. The acidic box is shown as AB within the open box. Dotted lines indicate sequence deletions and the heparin binding site is shown as HBS. (B) Binding activity of decoy receptor fusion proteins. The 96-well plates were precoated with 50 ng ml−1 FGF-2 in the absence (open squares) or presence (filled squares) of 100 ng ml−1 heparin at 4 °C overnight, after which conditioned media from CHO cells either as a negative control (NC) or containing 100 ng ml−1 decoy receptor fusion protein was added and followed by incubation with a horse radish peroxidase (HRP)-conjugated secondary antibody. The resulting absorbance was detected at 450 nm after adding HRP substrate and stop solution. The colour reproduction of this figure is available on the British Journal of Cancer journal online.