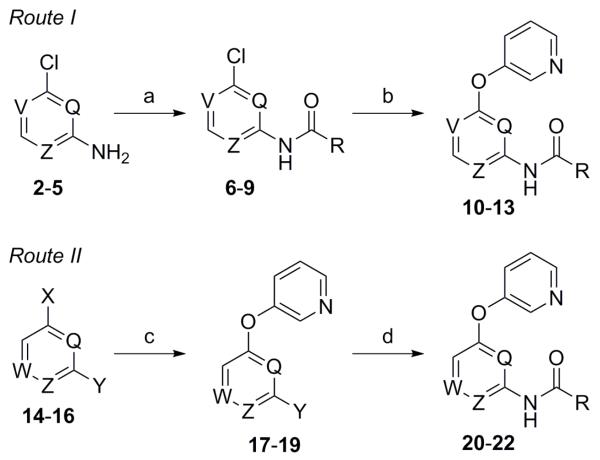
Scheme 1. Reagents and conditions: (a) For 2 (Z = N; V = Q = CH), 3 (V = N; Q = Z = CH), 4 (Q = N; V = Z = CH), and 5 (V = Z = N; Q = CH); RCO2H (R = 3-chlorophenyl), EDC, DMAP, CH2Cl2, (83–94%); (b) 3-hydroxypyridine, CuI, Cs2CO3, Me2NCH2CO2H·HCl (35–55%); (c) For 14 (W = N; Q = Z = CH; X = F; Y = Br), 15 (Q = W = N; Z = CH; X = Y = Cl), and 16 (Q = Z = N; W = CH; X = Y = Cl); 3-hydroxypyridine, K2CO3, DMF, microwave, 150 °C (60–93%); (d) RCONH2 (R = 3-chlorophenyl), NaOtBu, Pd(OAc)2, Xantphos, PhMe, 100 °C (50-78%).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.