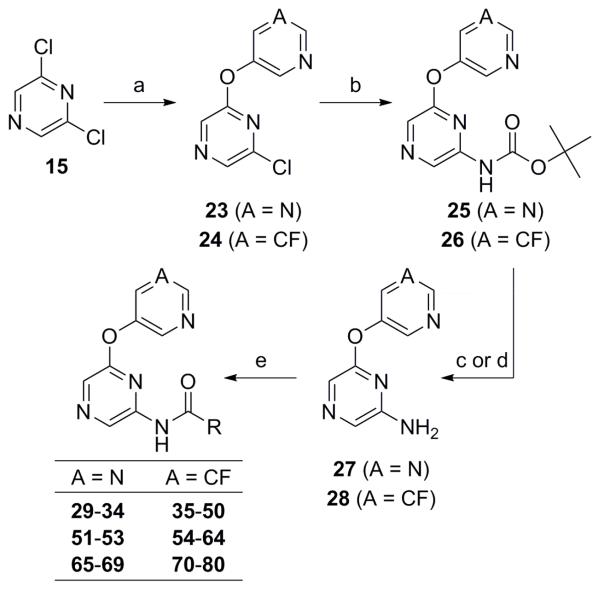
Scheme 2. Reagents and conditions: (a) 3-fluoro-5-hydroxypyridine or 5-hydroxypyrimidine, K2CO3, DMF, microwave, 120 °C (51% for 23; 62% for 24); (b) H2NCO tBu, NaO2 tBu, Pd2(dba)3·CHCl3, tBuXPhos, PhMe (46% for 25; 64% for 26); (c) For 25 27, TFA, CH2Cl2 (96%); (d) For 26 28, 4N HCl in dioxane (100%); (e) RCO2H, EDC, DMAP, CH2Cl2 or RCO2H, HATU, DIEA, CH2Cl2, DMF or RCO2H, POCl3, pyridine or RCOCl, DMAP, CH2Cl2 (30–80%).

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.