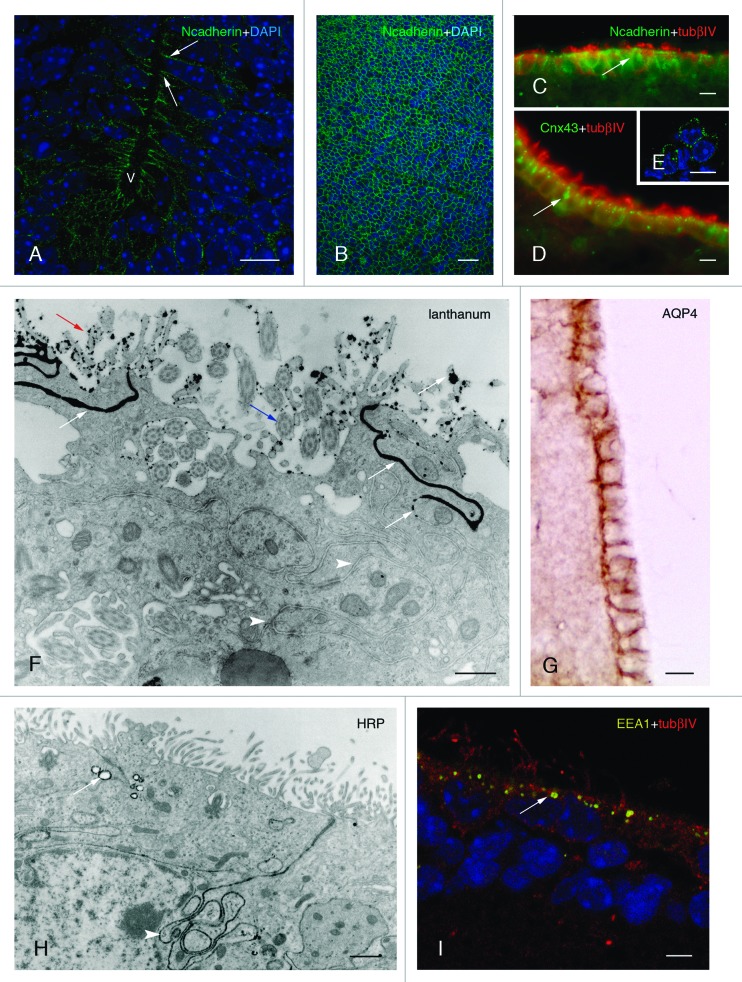
Figure 2. Development and properties of the multiciliated ependyma in the ventricle of the mouse. (A, B, and C). The neuroepithelial (A and B) and ependymal (C) cells express N-cadherin-containing junctions (in green, arrows) in their lateral plasma membrane domains, which are detected in transversal (A) and tangential views (B). (D and E) Multiciliated ependymal cells are joined with connexin43-containing (Cnx43) gap junctions (in green, arrow) that are appreciated in transversal (D) and tangential (E) views. (F) Multiciliated ependymal cells lack tight junctions, as shown with lanthanum nitrate applied to the ventricle and observed under transmission electron microscopy. The tracer (with black electrodensity, white arrows) is present passing through the lateral winding extracellular spaces (white arrowheads), proving the absence of functional tight junctions. Motile cilia (blue arrow) and microvilli (yellow arrow) are appreciated in the luminal pole of ependymocytes. (G) Aquaporin 4 (AQP4) is present in the laterobasal domain of multiciliated ependyma. (H) At the transmission electron microscope, multiciliated ependyma takes HRP applied in vivo into the ventricle, and the tracer is incorporated into the pynocytic vesicles and early endosomes (in black electrodense reaction, white arrow). The tracer is also observed in the lateral winding extracellular spaces (white arrowhead). (I) Early endosomes (detected with EEA1 in yellow, white arrow) are detected at the apical pole of multiciliated ependyma. (A and B) Micrographs represent Z-plane projections under confocal microscopy in 40-µm-thick frozen sections. (E and I) Micrographs represent 1 µm thick planes under confocal microscopy. (C and D) Micrographs are taken under fluorescent microscopy in a 10-µm-thick paraffin sections. Tubulin βIV (tubβIV) immunofluorescence is shown (in red) in cilia labeling in C and D. (A, B, E, and I) Micrographs present DAPI nuclear immunostaining (in blue). Abbreviations: v, ventricle lumen. Bars: A, C-E, G, 10 µm; B, 40 µm; F, 50 nm; H, 1 µm, I, 5 µm.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.