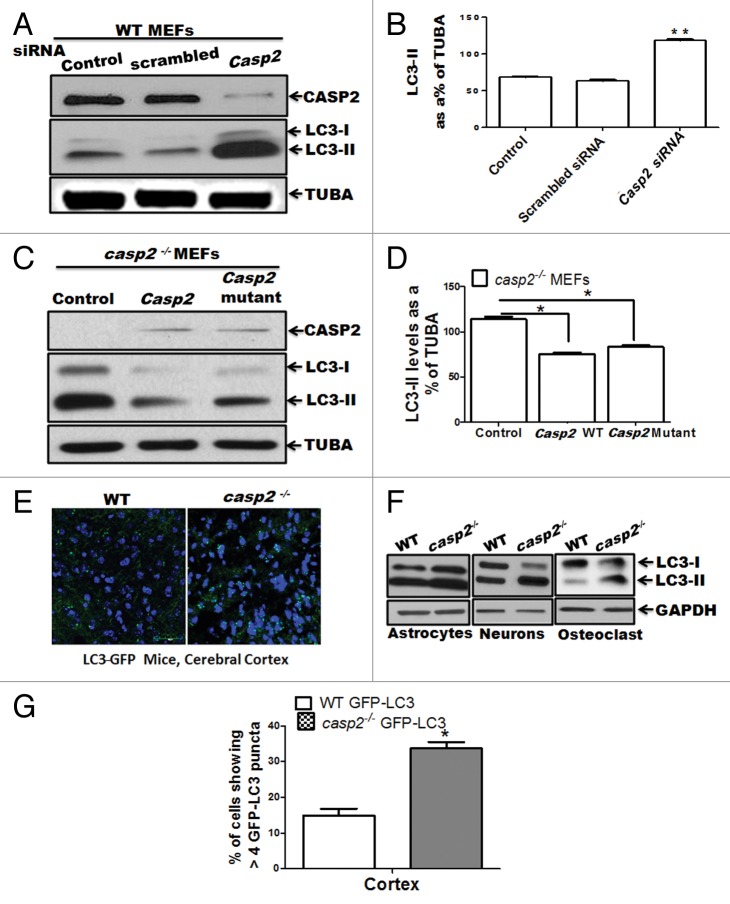
Figure 2.Casp2 knockdown or reinsertion can modulate autophagy. (A and B) WT MEFs were transiently transfected with prevalidated siRNA against Casp2. (C and D) casp2−/− MEFs were transiently transfected with Casp2 expression vector. (A and C) Western blot demonstrating the expression of CASP2 and autophagy was detected by determining the levels of LC3-II. The same blots were reprobed for determining tubulin, α (TUBA) levels that served as loading control. Shown are the representative blots. (B and D) Densitometric analysis for the level of expression was performed using ImageJ analysis. Expression levels of LC3-II were normalized by tubulin, α and expressed as LC3-II levels, as % of loading control. Each experiment was repeated at least 3 times. Error bar represents ± SEM. Statistical significance was determined by the Student t test. **P ≤ 0.01, *P ≤ 0.05. (E) Fluorescence confocal microscopy analysis mice of cerebellar sections from GFP-LC3 transgenic mice crossed with WT and casp2−/− mice. Images were acquired at 60X magnification. At least 12 to 13 images were analyzed to detect LC3 puncta formation that indicates autophagy. Shown is the percent of cells with more than 8 detectable LC3 puncta per image. Level of significance was determined by performing the Student t test, n = 12 images from 2 different mice. *P < 0.01. (F) Primary culture from astrocytes, neurons, and osteoclasts were established from WT and casp2−/− mice. The protein samples were prepared and western blotting for LC3 was performed to detect autophagy. Western blots for LC3 levels (an increase in LC3-II indicates an increase in autophagy) in casp2−/− cells compared with the WT. The same blots were reprobed for GAPDH that served as the protein loading control.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.