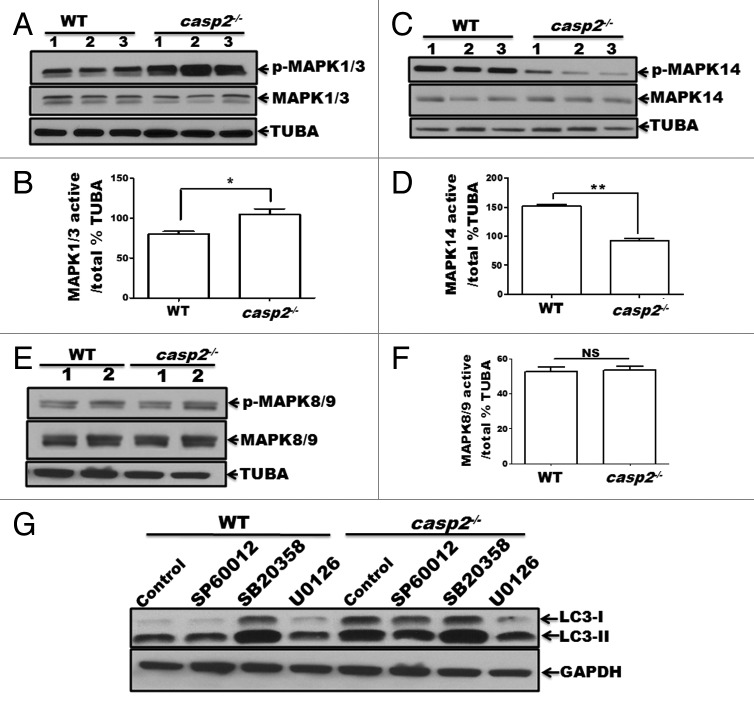
Figure 5. Role of MAPKs in CASP2-mediated modulation of autophagy. (A–F) Protein lysates were prepared from different batches of WT and casp2−/− MEFs (loaded in separate lanes 1 to 3 or 1 and 2 for MAPK8/9), cultured in complete medium in the absence of stressors. Western blotting was performed to detect the levels of active (phosphorylated) and total (non-phosphorylated) MAPKs using specific antibodies against (A) p-MAPK1/3, MAPK1/3 (C) p-MAPK14, MAPK14 and (E) p-MAPK8/9, and MAPK8/9 as described in the Materials and Methods. The same blots were reprobed for tubulin, α (TUBA) that served as a loading control. Shown are the representative blots. (B, D, and F) Densitometric analysis was performed using ImageJ to detect the expression levels. Y-axis represents expression levels of active vs. total MAPK as percent of the loading control. Error bar represents ± SEM. Statistical significance was determined by the Student t test, comparing WT vs. casp2−/−. **, P ≤ 0.01, *, P ≤ 0.05, NS, P ≥ 0.05, experiments repeated at least 3 times. (G) Effects of MAPK inhibitors: SP600125 (10 µM), SB203580 (10 µM), and U0126 (20 µM) on CASP2-mediated autophagy modulation. WT and casp2−/− MEFs were incubated with these inhibitors for 24 h followed by PepA (1 µM) and EST (10 µM) treatment for 6 h to determine autophagy flux. Autophagy was detected by western blotting to determine the levels of LC3 (LC3-I and LC3-II). GAPDH was used as a loading control. Shown is a representative blot, the experiment was repeated 3 times.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.