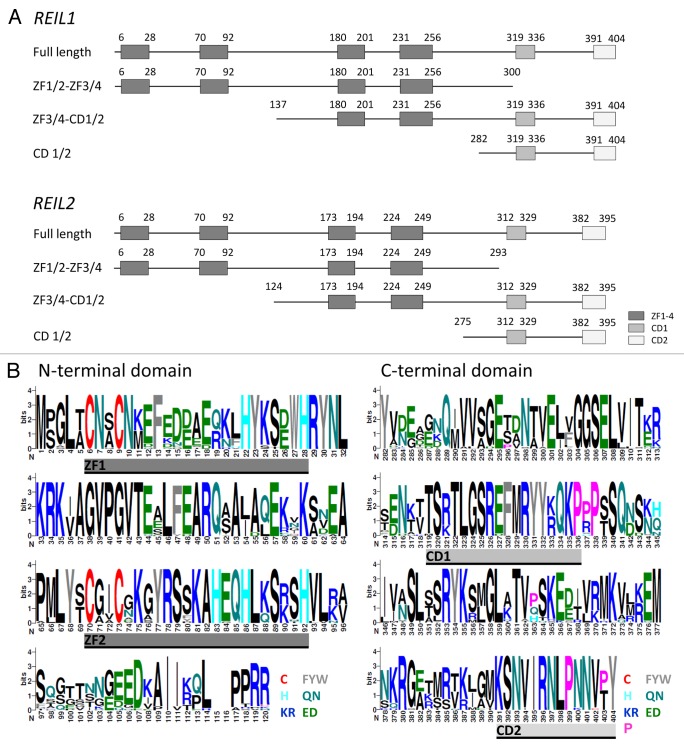
Figure 3. Topology of the REIL (Rei1-like) proteins from A. thaliana and of the partial proteins which were analyzed in this study by yeast-2-hybrid assays (Table 1).(A) The 404 amino acid REIL1 and the 395 amino acid REIL2 protein each contain 4 zinc finger domains and 2 additional conserved domains, provisionally named CD1 and CD2. The truncated proteins, ZF1/2-ZF3/4, contain the 4 zinc finger domains. The CD 1/2 truncations contain only the C-terminal parts with the conserved domains CD1 and CD2. The ZF3/4-CD1/2 partial proteins lack the N-terminal pair of zinc finger domains. (B) N-terminal and C-terminal alignment of REIL1 and REIL2 homologs of Brassicales species. Zinc finger domains, ZF1 and ZF2, and conserved domains, CD1 and CD2, are indicated. The color coding of the sequence logos highlights cysteine and histidine residues, aromatic amino acids, amide, acidic, basic residues, and the conserved proline residues of the C-terminus. Color coding is given with the insert. Sequence logos were generated at http://weblogo.berkeley.edu/logo.cgi after alignment of the REIL proteins from Arabidopsis thaliana, Arabidopsis lyrata subsp. lyrata, Eutrema parvulum, Eutrema salsugineum, Brassica rapa subsp. pekinensis, Capsella rubella, Camelina sativa, Leavenworthia alabamica, Sisymbrium irio, and Aethionema arabicum.

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.