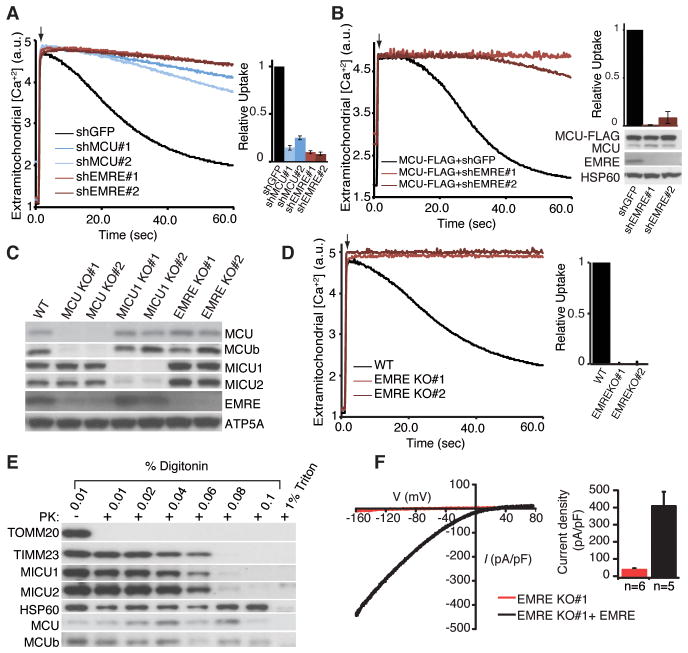
Fig. 3. Requirement of EMRE for uniporter activity.
(A) EMRE knock down impairs mitochondrial calcium uptake. Digitonin permeabilized HEK-293T cells were incubated with the calcium indicator Oregon Green Bapta 6F (OGB6F). After addition of 50 μM CaCl2 (arrow), the depletion of extracellular calcium due to mitochondrial uptake was monitored by OGB6F fluorescence. Representative traces of mitochondrial calcium uptake from HEK-293T cells with GFP (control), MCU and EMRE knock down are shown. The bar graph shows the rate of calcium uptake relative to shGFP cells (mean ± s.d., n=4). (B) MCU overexpression cannot rescue mitochondrial calcium uptake in EMRE knock down cells. EMRE was knocked down in HEK-293T cells that overexpress MCU-FLAG. The samples were treated as in (A) (n=4). Lysates were analyzed by immunoblotting for indicated proteins. (C) Loss of MCU decreases EMRE and MCUb abundance. MCU, MICU1 and EMRE knockout (KO) HEK-293T cells were generated using TALEN technology, cell lysates from wild type (WT) and two independent knockout clones were prepared and analyzed by immunoblotting for control ATP5A and uniplex proteins (D) Mitochondrial calcium uptake is severely impaired in cells that lack EMRE. Mitochondrial calcium uptake was measured in WT and two independent EMRE KO cell lines as in (A). (E) Loss of EMRE does not change MCU, MCUb, MICU1 and MICU2 submitochondrial localization. Mitochondria were isolated from cells lacking EMRE, incubated with increasing concentrations of digitonin in the presence of proteinase K (PK), samples were analyzed by immunoblotting for outer membrane protein TOMM20, inner membrane protein TIMM23, matrix protein HSP60, as well as uniplex proteins (F) Exemplar trace (red) of a mitoplast derived from EMRE knockout cells demonstrates absent calcium current (IMiCa), whereas typical IMiCa is seen after EMRE is knocked back in (black). Voltage ramps were delivered from −160mV to +80mV for 750ms, using a holding potential of 0mV. (Right) Summary data. Error bars report SEM.