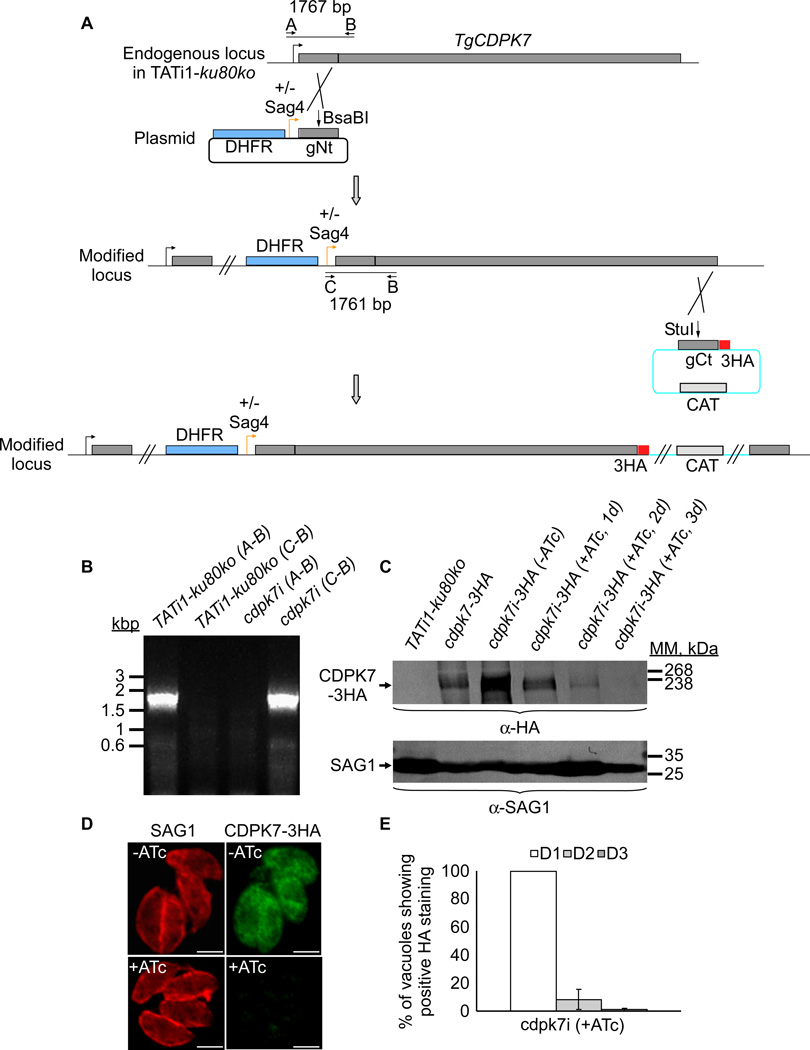
Figure 2. TgCDPK7 conditional knock-in by promoter exchange strategy.
(A) Schematic representation of the strategy used to replace the endogenous promoter of TgCDPK7 with the tetracycline inducible promoter. The DHFR-tetO7-SAG4NtgCDPK7 plasmid contains the dihydrofolate reductase (DHFR) gene (in blue) and the N-terminal genomic coding sequence of TgCDPK7 (in grey, 1455 bp) under the control of the inducible tetO7SAG4 promoter (Orange arrow). Black arrows represent the primers used for PCR analysis and the length of the PCR fragments generated is indicated. To study the regulation of TgCDPK7i gene, we inserted by single homologous recombination at the 3’ of the gene, a sequence coding for three HA epitope tags at the C-terminus of the corresponding TgCDPK7i protein. (B) PCR analysis performed on Tgcdpk7i, showing that single homologous recombination occurred. Genomic DNA from TATi1-ku80ko parasites was used as negative control. (C) Western blot analysis of TATi1-ku80ko and cdpk7i-3HA strains in presence or in absence of ATc. Parasites were treated for 1 or 2 or 3 days (d) with ATc. SAG1 was used as loading control. (D) Down regulation of TgCDPK7-3HA in the cdpk7i strain as shown by IFA with anti-HA antibodies 3 days after ATc treatment. Scale bars represent 2 µm. (E) Quantification of the percentage of vacuoles showing positive HA staining upon treatment with ATc at days 1 or 2 or 3 respectively using anti-HA antibodies.