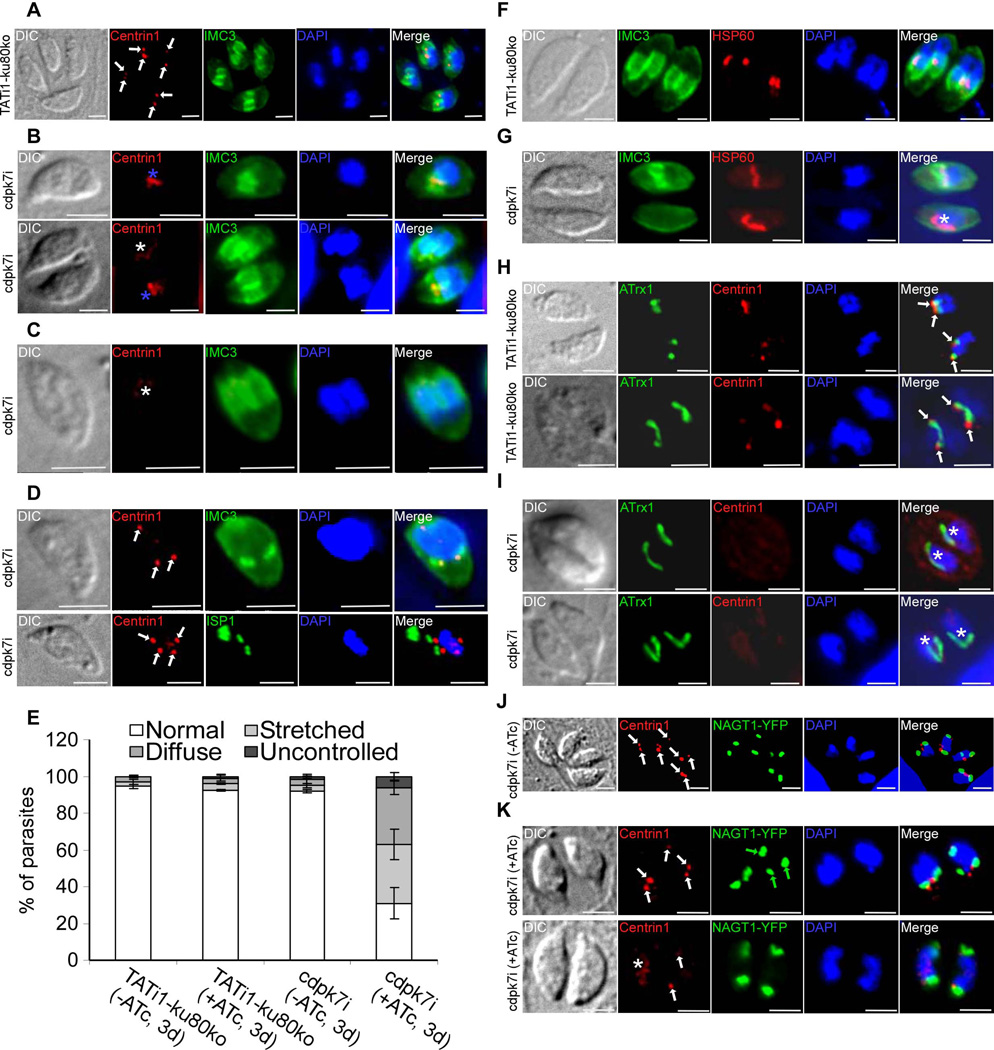
Figure 6. cdpk7i mutant parasites display centrosomal defects, reveal a plastid elongation independent of centrin 1 protein and IMC buds and show the dependency of the Golgi positioning and number with that of centrosome duplication.
A) to (D): IFA analysis of parasites undergoing division after 3 days of ATc treatment. (A) to (D): anti-centrin1 and anti-IMC3 or anti-ISP1 antibodies were used to stain the centrosome and daughter buds, respectively. (A) A representative TATi1-ku80ko vacuole containing 4 parasites is shown. The duplicated centrosomes (in red) in each parasite are marked with a white arrow. Each newly formed IMC (in green) encapsulates one centrosome. (B) to (D): Representative examples of cdpk7i parasites showing centrosomes or centrin 1 staining defects. (B) A stretched centrosome (blue asterisks) which is located in between two newly formed daughter buds (upper image) or partitioned without being split between the nascent IMCs (lower image) is shown. (C) A diffused centrin 1 staining (white asterisk) is shown. (D) Two parasites in division showing an abnormal number of centrosomes per nucleus (white arrows) (3 in the upper image and 4 in the lower picture). (E) Scoring of centrosome defects during parasite division by IFA using anti-centrin1 antibodies. Interference with TgCDPK7 function led to stretched centrosomes, affected the distribution of centrin 1 protein and impaired the regular number of centrosome duplication. Data are mean values ± SD for three independent experiments. (F) to (I): IFA analysis of vacuoles containing 2 parasites undergoing division after 3 days of ATc treatment. (F) and (H): IFAs of representative TATi1-ku80ko vacuoles which were compared to IFAs of representative cdpk7i vacuoles, (G) and (I). (F) and (G): anti-IMC3 and anti-HSP60 antibodies were used to stain the daughter buds and the apicoplast, respectively. (F): a TATi1-ku80ko vacuole with newly formed IMC (in green) encapsulating each apicoplast. (G) A cdpk7i vacuole exhibiting the formation of IMC buds in only one parasite out of two. In the parasite lacking the 2 IMC buds, the apicoplast adopts its elongated shape and lies on the nucleus (white asterisk). (H) and (I): anti-centrin 1 and anti-ATrx1 antibodies were used to stain the centrosome and the apicoplast, respectively. (H) The ends of the plastid in dividing apicoplasts of the TATi1-ku80ko parasites are consistently associated with the parasite’s centrosomes. This association is maintained even after apicoplast division (white arrows). (I) Two representative cdpk7i vacuoles showing the diffused staining of centrin 1 protein. In the parasite lacking a concentrated centrin 1 dotted-like staining, the apicoplast adopts its elongated shape and lies on the nucleus (white asterisk). (J) and (K): anti-centrin 1 antibodies and NAGT1-YFP were used to detect the centrosome and the Golgi, respectively. (J) The two centrosomes (white arrows) are found at the inner ends of the newly-divided Golgi (in green). (K) Two representative cdpk7i vacuoles treated with ATc showing an abnormal numbers of centrosomes and Golgi per nucleus (white and green arrows, upper image), and a diffuse staining of centrin 1 protein (white asterisk) or two centrosomes in up and down position (white arrows) (lower image). Scale bars represent 2 µm.