Figure 1.
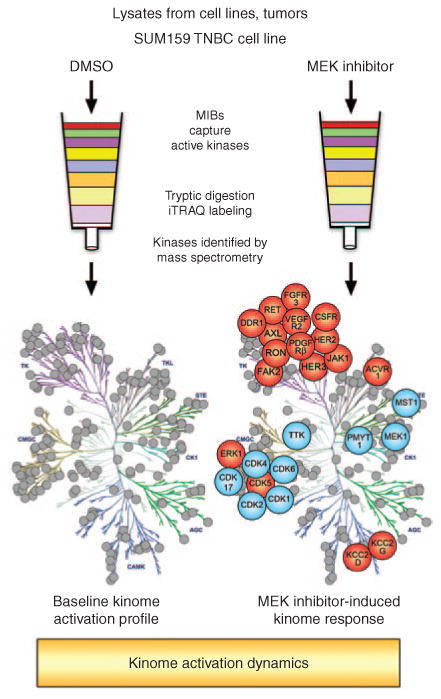
Multiplexed kinase inhibitor beads coupled with mass spectrometry (MIB/MS) as a technique for whole-kinome activation profiling. Total protein lysates from cell lines, tumors, or other tissues are flowed over affinity columns composed of MIBs: custom-synthesized, linker-adapted small-molecule kinase inhibitors covalently attached to Sepharose beads. Multiple type I kinase inhibitors are used to enrich for active kinases from every branch of the kinome tree. Kinases are eluted from the column and digested to generate a complex peptide mixture. Peptides are separated by liquid chromatography and identified by mass spectrometry. Labeling with isobaric tags for relative and absolute quantitation (iTRAQ) allows multiple samples to be compared in a single mass spectrometry run. In this manner, a 24-hour treatment of the SUM159 triple-negative breast cancer (TNBC) cell line with a MEK inhibitor was compared with dimethyl sulfoxide (DMSO) control–treated cells. MEK inhibition generates global changes in kinase activation. In particular, multiple tyrosine kinases demonstrate increased activity (in red) following MEK inhibitor treatment (blue indicates loss of activity).
