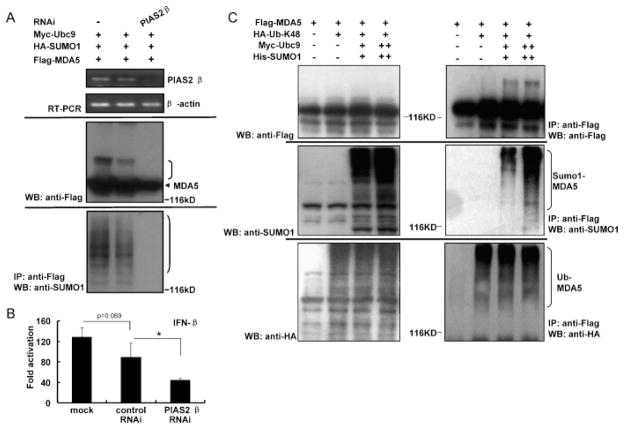
Fig. 5.
PIAS2β knockdown inhibits the SUMOylation of MDA5 and the IFN response. (A) Downregulation of PIAS2β inhibits SUMOylation of MDA5. HEK293T cells were transiently transfected with siRNA sequence targeting the human PIAS2β gene or its scrambled control. Twelve hours later, cells were transfected with plasmids encoding Flag-MDA5, Myc-Ubc9 and HA-SUMO-1 and incubated for 36 h. RT-PCR analysis (left panel) shows the knockdown efficiency of endogenous PIAS2β. β-Actin was detected as an internal control. In parallel, SUMOylation of MDA5 was assessed as in Fig. 1A (middle and right panels). (B) Downregulation of PIAS2β inhibits MDA5-driven IFN-β reporter activities. Twenty-four hours after PIAS2β knockdown as in (A), cells were transfected with plasmids for renilla plasmid, IFN-β reporter and Flag-MDA5 plasmids for an additional 24 h. Calculation of fold activation and statistical analysis were as in Fig. 4D. (C) SUMOylation does not affect the Lys48-linked poly-ubiquitination of MDA5. HEK293T cells were transiently transfected with plasmids encoding Flag-MDA5, Myc-Ubc9, His-SUMO-1 and HA-Ub-K48 for 48 h. Whole cell lysates were immunoprecipitated with anti-Flag antibody followed by immunoblotted with anti-Flag, anti-SUMO1 or anti-HA antibody. Protein expression levels were detected with anti-Flag, anti-SUMO1 or anti-HA antibody, respectively.