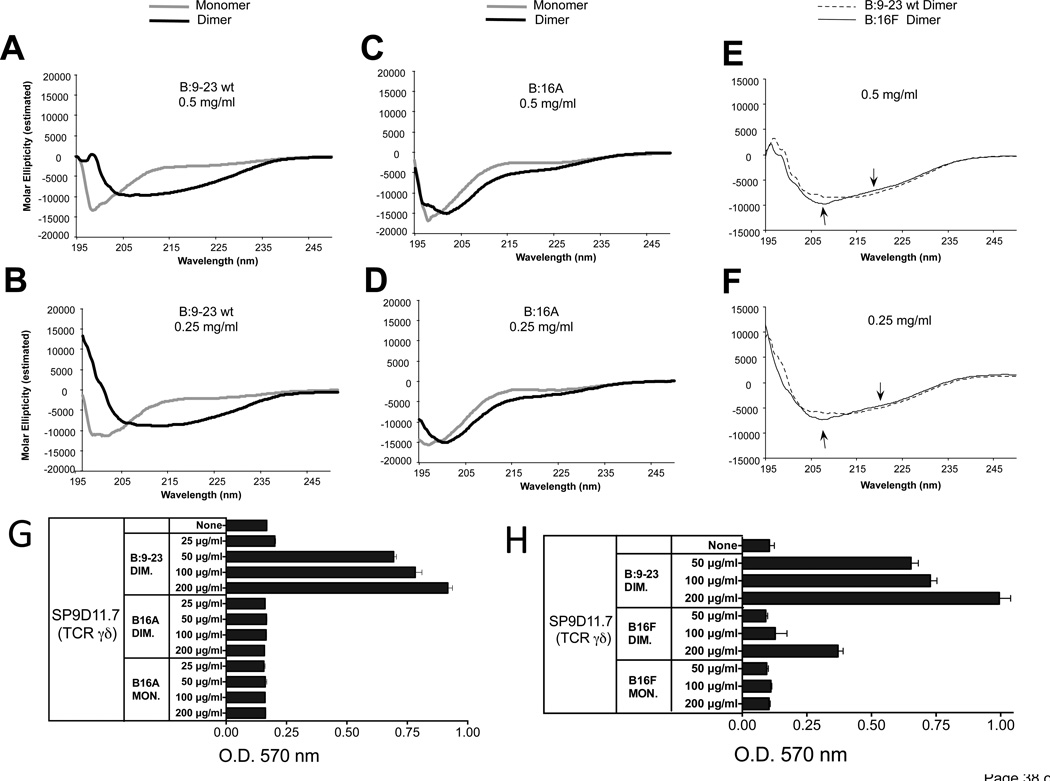
Figure 9. As an oxidized dimer, the B:9-23 peptide antigen adopts a distinct secondary structure, which requires Tyr16.
Panels A, B CD spectra of monomeric and oxidized dimeric B:9-23 peptides (wild-type)
Panels C, D CD spectra of monomeric and oxidized dimeric B16A peptides
Panel E, F Comparison of CD spectra of oxidized dimeric wild-type and B16F peptides
CD spectroscopy was performed on HPLC-purified monomeric and dimeric B:9-23 wild-type (wt) (panels A and B) and amino-acid substituted peptides (panels C–F), at two peptide concentrations. Dimerization induces a shift in the circular dichroism of the wt peptide consistent with a change from a random structure of the monomer to a beta-pleated sheet structure of the dimer (panels A, B). In contrast, dimerization does not substantially change the circular dichroism of the B:16A substituted peptides (panels C, D). The dimeric B16F substituted peptide does shift in circular dichroism, but slightly less than the dimeric wt peptide (see arrows in panels E, F).
Panel G The B16A substituted peptide fails to stimulate a response of γδ hybridoma SP9D11
Culture conditions and response measurements using the LacZ assay were as described in Fig.1.
Panel H The B16F substituted peptide elicits a smaller response of γδ hybridoma SP9D11 than the wt peptide
Culture conditions and response measurements using the LacZ assay were as described in Fig.1.