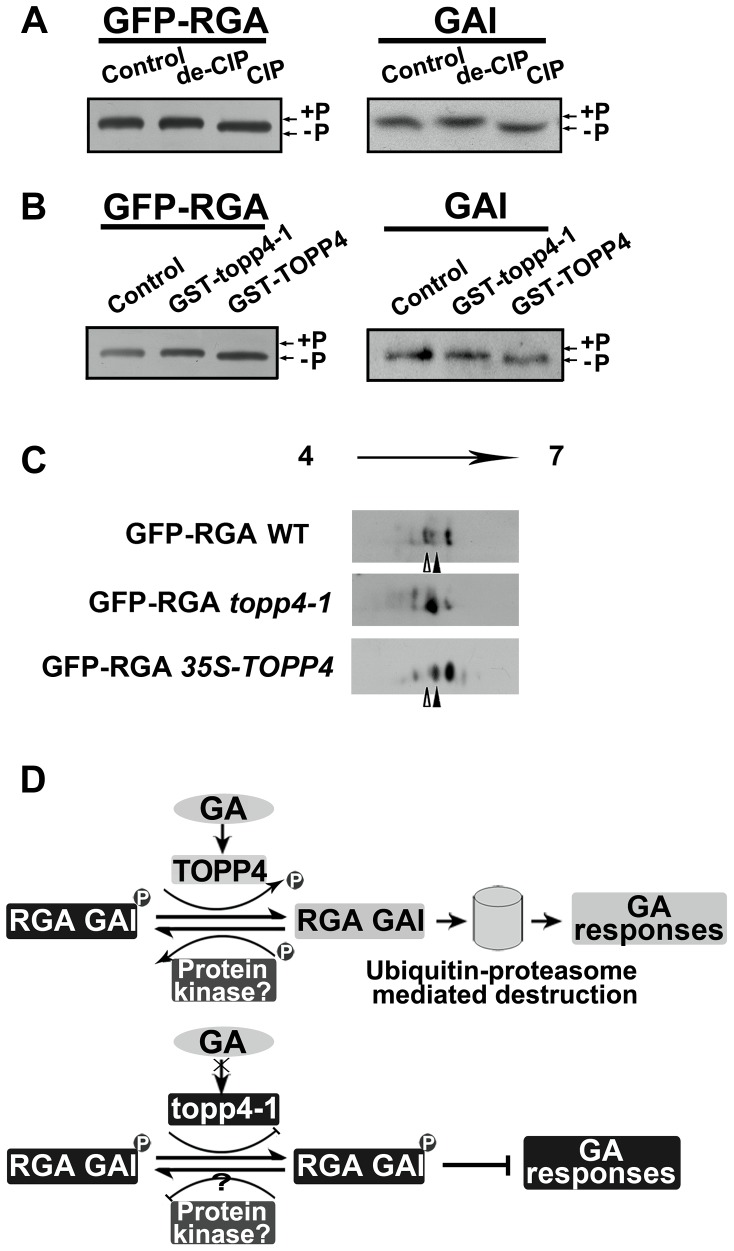
Figure 10. DELLA proteins are direct substrates of TOPP4.
(A) Immunoblotting assays of GFP-RGA and GAI proteins incubated with denatured CIP (de-CIP) or CIP. (B) Immunoblotting assays of GFP-RGA and GAI proteins incubated with GST-topp4-1 or GST-TOPP4 from E. coli suggested that TOPP4, but not topp4-1, can directly dephosphorylate phosphorylated RGA and GAI. Phosphorylated status, +P; dephosphorylated status, −P. (C) 2-DE analyses of post-translational modification of GFP-RGA in wild-type, topp4-1, and TOPP4 overexpressing plants. The phosphorylation status of RGA was increased in topp4-1 while the dephosphorylation status of RGA was increased in 35S-TOPP4 plants compared to wild type. The total protein extractions were separated by 2-DE and immunoblotted with anti-GFP antibody. (D) A current model of TOPP4 function on DELLA stability in the GA signaling pathway.