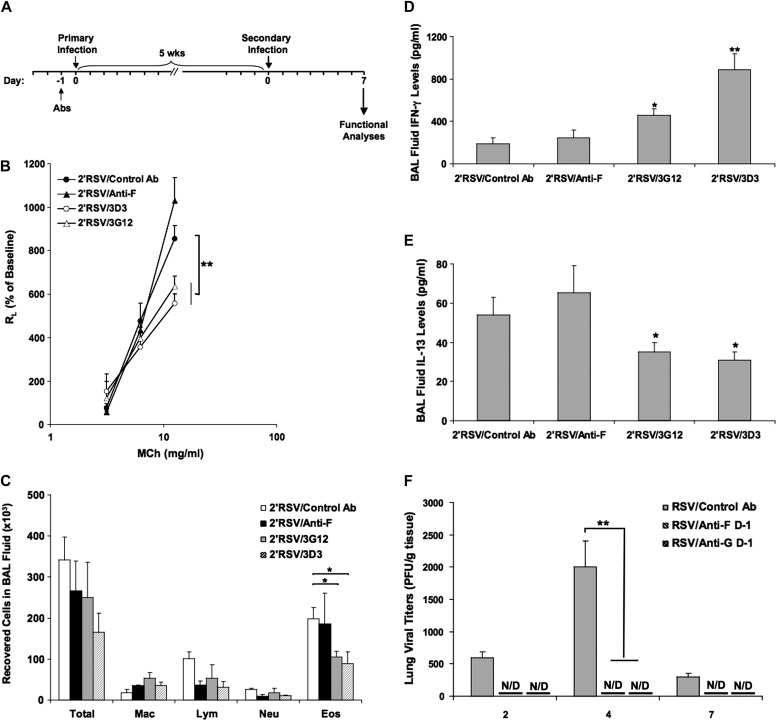
Figure 4.
Anti–RSV-G mAb reduces the response to secondary RSV infection when administered during neonatal primary infection. (A) Experimental protocol. (B) Airway responsiveness to inhaled MCh. (C) BAL cellularity. (D) BAL fluid IFN-γ levels. (E) BAL fluid IL-13 levels. (F) Virus titers in the lung after primary neonatal RSV infection. Results from three independent experiments with 12 mice per group are expressed as mean ± SD. *P < 0.05, **P < 0.01 indicate significant differences between anti–RSV-G antibody-treated mice and control antibody-treated mice. N/D, not detected.