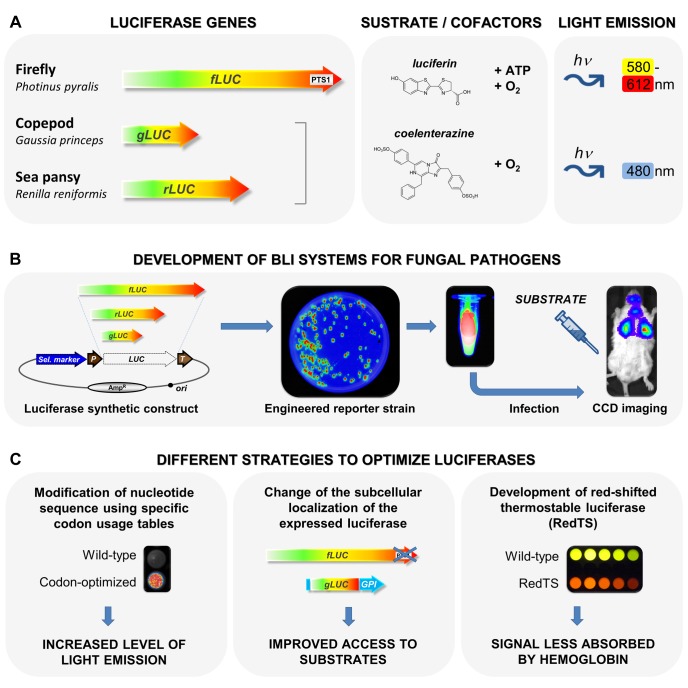
Figure 1. Development and optimization of bioreporters for fungal infection BLI.
(A) The three luciferase-encoding genes used in Aspergillus and Candida species. The firefly (Photinus pyralis) fLUC gene encoding a luciferase (Fluc), which converts the substrate luciferin to oxy-luciferin in an ATP-dependent manner, the sea pansy (Renilla reniformis) rLUC and the copepod Gaussia (Gaussia princeps) gLUC, which both encode luciferases (Rluc and Gluc, respectively) that produce light emission from the substrate coelenterazine. All luciferases require oxygen for activity. (B) Schematic presentation of the different steps of the construction of fungal bioreporters and their use in BLI. A fungal strain is first genetically modified to stably express a luciferase gene as a reporter. Luciferase-expressing cells are used for animal infection and the substrate, luciferin or coelenterazine, is extemporaneously administered. Finally, light emitted from reporter cells is externally monitored in real time, using sensitive photon detectors based on cooled or intensified charge coupled devices (CCD). (C) Compilation of various strategies to optimize luciferases used in fungal infection BLI. This includes the adaptation of luciferase nucleotide sequences to the fungal host using specific codon usage tables (left panel), the change of the subcellular localization of the expressed luciferase either by removing (PTS1 of firefly luciferase) or by adding (GPI anchor for Gaussia luciferase) targeting sequences (middle panel), and the generation of red-shifted/thermostable firefly luciferases through enzyme redesign (right panel).