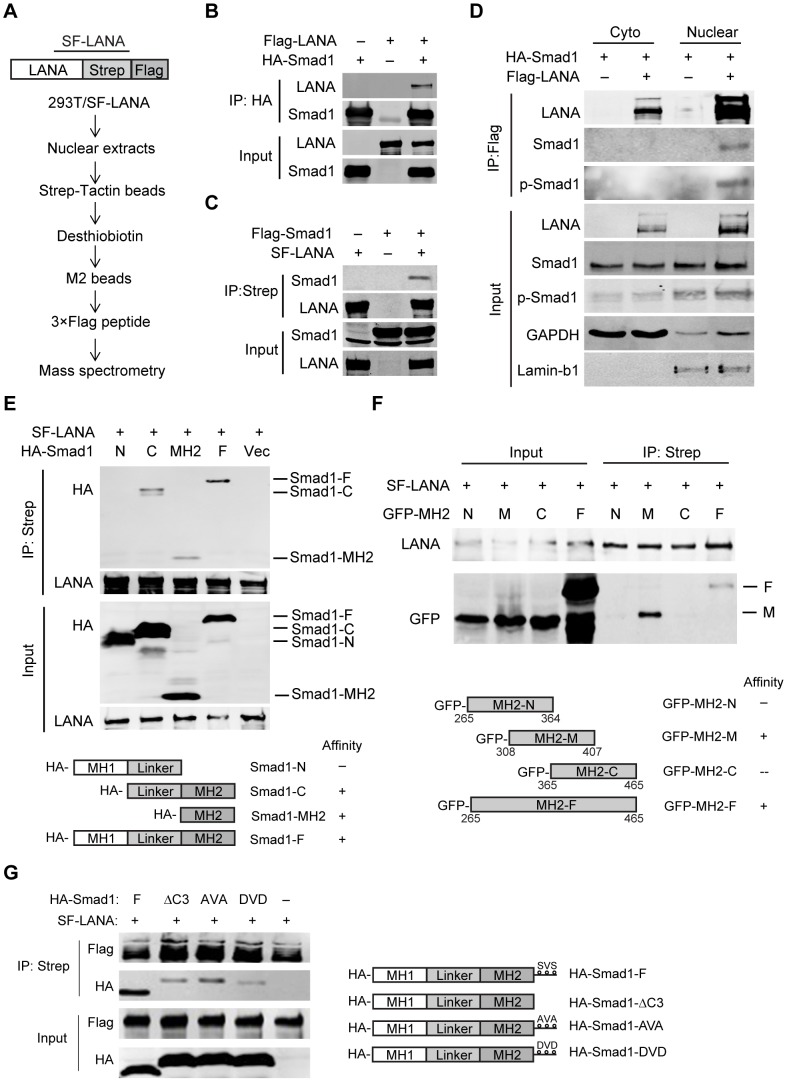
Figure 1. LANA interacted with BMP-activated Smad1 in the nucleus.
(A) Schematic strategy showing tandem affinity purification of SF-tagged LANA Complex. (B, C) Reciprocal co-immunoprecipitation assays testing physical interactions between LANA and Smad1. Flag-LANA and HA-Smad1 (12 µg each, B), or Flag-Smad1 and SF-LANA (12 µg each, C) were co-transfected into 293T cells. Cells were lysed for co-immunoprecipitation as indicated. (D) LANA interacted with BMP-activated p-Smad1 in the nucleus. Flag-LANA and HA-Smad1 (12 µg each) were co-transfected into 293T cells. After serum starvation overnight, cells were treated with BMP2 for 2 hours, and then cells were fractionated and subjected to co-IP assay as indicated. (E) LANA interacted with Smad1 MH2 domain. Different truncated Smad1 constructs were co-transfected with SF-LANA (12 µg each) into 293T cells. Cell lysates were immunoprecipitated as indicated. (F) LANA interacted with center part of Smad1 MH2 domain. Different truncated Smad1-MH2 constructs were co-transfected with SF-LANA (12 µg each) into 293T cells. Cell lysates were immunoprecipitated as indicated. (G) LANA-Smad1 interaction did not depend on the phosphorylation of SXS motif of Smad1. HA-tagged full length Smad1 or SXS motif truncated ΔC3 mutant, or SXS motif inactivated mutant AVA, or SXS motif activated mutant DVD or vector were cotransfected with SF-LANA (12 µg each) into 293T cells. Cell lysates were immunoprecipitated as indicated.