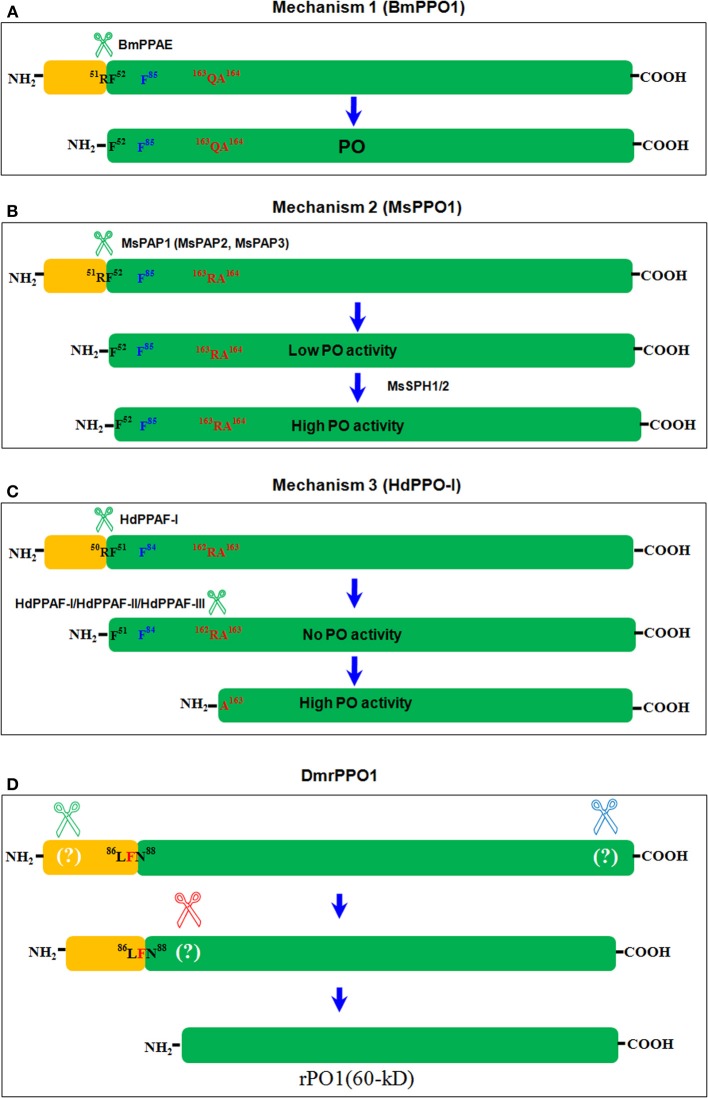
Figure 1.
Three mechanisms of insect PPO activation. The terminal serine protease cleaves PPO differently among insect species (Ashida and Brey, 1997; Lee et al., 1998; Kim et al., 2002; Kanost and Gorman, 2008). And the three mechanisms diverge based on these differences. Here, we consider only BmPPO1 (Accession: NP_001037335), MsPPO1 (Accession: O44249), and HdPPO1 (Accession: BAC15603) as a summary. The conserved bonds 51RF52 in BmPPO1 (A), 51RF52 in MsPPO1 (B), and 50RF51 in HdPPO1 (C) were cleaved by serine proteases separately in the same way, as shown. F85 in BmPPO1, F85 in MsPPO1, and F84 HdPPO1 (labeled in blue) function as the place holder in the respective active site pockets, and may block substrates until they are dislodged. In HdPPO1, 162RA163 was further cleaved to form a fragment at 60 kD with PO activity (C). The corresponding sequences in BmPPO1 (A) and MsPPO1 (B) are also shown in red. In B. mori, BmPPO was cleaved by PPAE to produce PO (Ashida and Brey, 1997) (A). In M. sexta, PAPs cleaved MsPPO in the same place as in B. mori to produce PO fragments with low enzyme activity. When SPHs were added, PO activity increased significantly (Kanost and Gorman, 2008) (B). In H. diomphalia, HdPPO was cleaved as in BmPPO and MsPPO by PPAF-I at the conserved bond 50RF51. However, the large fragment had no PO activity unless PPAF-I, PPAF-II, and PPAF-III were combined, and was cleaved again at 162RA163 to produce a fragment at 60 kD; this fragment had PO activity (Lee et al., 1998; Kim et al., 2002) (C). In an in vitro assay, commercial α-chymotrypsin cleaved D. melanogaster recombinant PPO1 (Accession: AAF57775) (rPPO1) in at least three places to produce a fragment, also of ~60 kD, with direct enzyme activity (Lu et al., 2014a) (D).