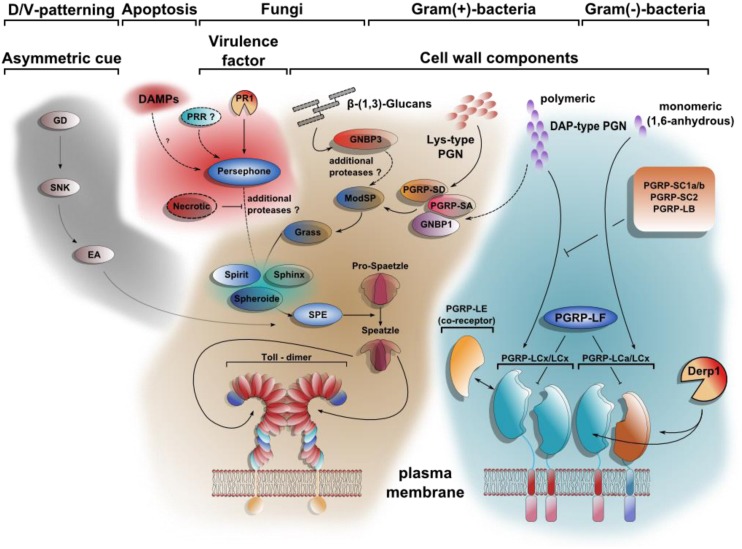
FIGURE 2.
Toll signaling integrates DAMPs and PAMPs. At least four different extracellular pathways activate Spätzle proteolytically. The first comprises a cascade of serine proteases, which defines dorso-ventral polarity in the developing egg. Gastrulation defective (GD), Snake (SNK), and Easter (Ea) induce the hydrolytic cleavage of Spätzle on the ventral side of the embryo. DAMPs can be sensed by Persephone (Psh) as shown for a model with defects in apoptosis. The proteolytic cleavage of Psh by the fungal protease PR1 renders it active as well. The pattern recognition receptor GNBP3 (Gram negative bacteria binding protein 3) senses β-1,3 glucan-stretches from fungal cell walls parallel to PAMPs. The Toll pathway is also required for resistance toward Gram-positive bacteria due to the recognition of polymeric Lys-type PGN by PGRP-SA, PGRP-SD, and GNBP1. Whereas the downstream signaling of the Toll-activating pattern recognition receptors (PRRs) converge on the modular serine protease (ModSP), the Persephone-mediated Damage-signal will be integrated by the secreted Sphinx1/2-, Spheroide- and Spirit-serine proteases, resulting in the activation of the Spätzle-activating enzyme (SPE). In contrast to the extracellular multistep activation cascade of the Toll-pathway, the stimulation of Imd-signaling is achieved by direct binding of PGN to receptor dimers. PGRP-LE, PGRP-LF, PGRP-SC1a/b, PGRP-SC2, and PGRP-LB all share regulatory functions. Similar to Psh-activation by PR1-hydrolysis, PGRP-LC can be activated after cleavage by Der p 1 [modified after (Ferrandon et al., 2007; Lemaitre and Hoffmann, 2007)].