Abstract
Most chloroviruses encode small K+ channels, which are functional in electrophysiological assays. The experimental finding that initial steps in viral infection exhibit the same sensitivity to channel inhibitors as the viral K+ channels has led to the hypothesis that the channels are structural proteins located in the internal membrane of the virus particles. This hypothesis was questioned recently because proteomic studies failed to detect the channel protein in virions of the prototype chlorovirus Paramecium bursaria chlorella virus 1 (PBCV-1). Here, we used a mAb raised against the functional K+ channel from chlorovirus MA-1D to search for the viral K+ channel in the virus particle. The results showed that the antibody was specific and bound to the tetrameric channel on the extracellular side. The antibody reacted in a virus-specific manner with protein extracts from chloroviruses that encoded channels similar to that from MA-1D. There was no cross-reactivity with chloroviruses that encoded more diverse channels or with a chlorovirus that lacked a K+ channel gene. Together with electron microscopic imaging, which revealed labelling of individual virus particles with the channel antibody, these results establish that the viral particles contain an active K+ channel, presumably located in the lipid membrane that surrounds the DNA in the mature virions.
Introduction
Chloroviruses are members of a large, rapidly expanding genus (genus Chlorovirus, family Phycodnaviridae) of plaque-forming dsDNA viruses that infect certain unicellular, exsymbiotic, Chlorella-like green algae (Van Etten, 2003; Yamada et al., 2006; Van Etten & Dunigan, 2012). The viruses have large genomes (290–370 kb), are structurally similar, have an internal membrane surrounded by a glycoprotein coat and have considerable genetic diversity. Chloroviruses are present in freshwater environments all over the world, and titres as high as 100 000 infectious particles (ml indigenous water)−1 have been reported (Zhang et al., 2011). Chloroviruses are grouped according to their three known hosts: viruses that infect Chlorella variabilis (former name Chlorella NC64A; these viruses are referred to as NC64A viruses), viruses that infect Chlorella heliozoae (former name Chlorella SAG 3.83; these viruses are referred to as SAG viruses) and viruses that infect Micractinium conductrix (former name Chlorella Pbi; these viruses are referred to as Pbi viruses).
Genomic sequencing of 41 chloroviruses has established that 39 of them encode small (82–98 aa) K+-channel proteins (Jeanniard et al., 2013). Expression of several of these K+-channel-encoding genes in heterologous systems including Xenopus laevis oocytes (Plugge et al., 2000; Gazzarrini et al., 2006), mammalian human embryonic kidney HEK293 cells (Moroni et al., 2002), Chinese hamster ovary cells (Gazzarrini et al., 2003) and Saccharomyces cerevisiae (Balss et al., 2008) have established that these ion channels (named Kcv for K+ chlorovirus) are functional (Thiel et al., 2011). Electrophysiological assays indicate that the Kcvs have most of the functional properties of canonical K+ channels (Plugge et al., 2000; Thiel et al., 2011). Considerable experimental data support the hypothesis that the Kcv channels are packaged in the virions and that they play an important role in the initial phase of infection (Thiel et al., 2010). Circumstantial evidence that supports this hypothesis includes the following: (i) chloroviruses induce host-membrane depolarization and K+ is released from the host very early in the infection process (Neupärtl et al., 2008); (ii) virus-induced depolarization, release of K+ from the host and injection of DNA from the virus into the host have the same distinct sensitivity to ion-channel inhibitors as the conductance of the viral K+ channels in heterologous expression systems (Frohns et al., 2006); (iii) the Kcv genes are transcribed as late genes, and late gene products are often packaged in the virion (Kang et al., 2004); and (iv) the virus particles have an internal membrane, which must be intact for successful infection (Skrdla et al., 1984; Yan et al., 2000; Mehmel et al., 2003).
However, all attempts to detect Kcv channel protein associated with the prototype chlorovirus Paramecium bursaria chlorella virus 1 (PBCV-1) virion (KcvPBCV-1) have been unsuccessful. For example, a comprehensive proteomic study revealed that highly purified PBCV-1 particles contain 148 unique virus-encoded proteins and one host protein (Dunigan et al., 2012), but KcvPBCV-1 was not detected. Because membrane proteins are notoriously difficult to identify by mass spectroscopy (Fischer & Poetsch, 2006), we suspect that this small membrane-embedded protein might not be detected by mass spectrometry. To examine this paradox, we generated polyclonal and mAbs against the Kcv channel. These antibodies allowed us to detect the Kcv protein in the virions of some highly purified chloroviruses.
Results and Discussion
Kcv antibody
To test for Kcv protein in chlorovirus particles, we initially used a commercial polyclonal antibody against a peptide analogue to the Kcv ‘turret’ domain (FSVANPDKKA) from KcvPBCV-1. This domain on the extracellular side of the channel (Tayefeh et al., 2007), connects the first transmembrane domain, TM1, to the pore helix and is the only region in the protein with predicted antigenic potential. As expected, this antibody recognized purified KcvPBCV-1 in Western blots; the antibody also reacted with protein bands that were the size of the channel monomer and tetramer in extracts from disrupted PBCV-1 particles (results not shown). However, the antibody cross-reacted with other viral proteins that were not Kcv oligomers. Therefore, the antibody was not specific enough to determine whether the channel is present in chlorovirus particles.
To improve the specificity of the detection probe we produced a mAb (anti-Kcv-8D6) against the functional Kcv tetramer from chlorovirus MA-1D (KcvMA-1D). Fig. 1(a) shows that KcvMA-1D purified from Pichia pastoris by means of the N-terminal His tag had a molecular mass of ~42 kDa. This mass corresponds to the tetrameric form of the channel, which is very stable in SDS gels when unboiled samples are loaded on the gel. The monomeric form was only detected by boiling the sample for 10 min prior to loading (Fig. 1a, lane B) and demonstrated a size of ~10 kDa. The anti-Kcv-8D6 antibody clearly recognized the tetramer but not the monomer in a Western blot (Fig. 1a). After removing the tag with viral 3C protease, the tetramer ran at the expected molecular mass of 35 kDa (Fig. 1b), which was also recognized by the antibody (Fig. 1c).
Fig. 1.
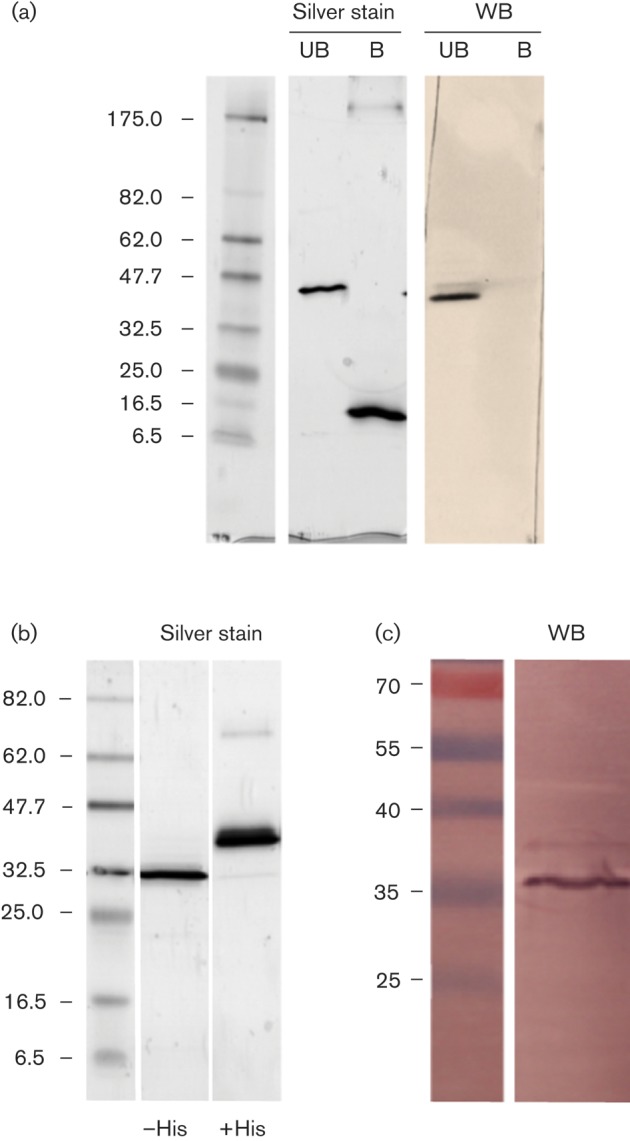
(a) SDS-PAGE separation of purified unboiled (UB) or boiled (B) KcvMA-1D with a His tag. Silver staining revealed a single band for the tetramer and for the monomer of unboiled and boiled protein, respectively. In a Western blot (WB) anti-Kcv-8D6 only recognizes the tetramer in unboiled samples. (b) KcvMA-1D with a His tag (+His) and after removing the tag (−His) in an SDS-PAGE gel stained with Coomassie blue. The tag-free channel formed a single band of ~35 kDa. (c) A Western blot of the tag-free KcvMA-1D protein using anti-Kcv-8D6 antibody detected a single band of ~35 kDa. Molecular size markers are shown on the left of blots (kDa).
Previous experiments established that K+ but not Na+ in the buffer stabilizes the KcvPBCV-1 tetramer (Pagliuca et al., 2007). To confirm that anti-Kcv-8D6 was reacting with the channel tetramer we performed experiments with either K+ or Na+ in the SDS buffer. The blot in Fig. 1(c) shows that anti-Kcv-8D6 reacted positively in the presence of 200 mM KCl with a 35 kDa protein. When KCl was replaced with NaCl, the antibody showed no binding (see Fig. 4c). These results supported the conclusion that anti-Kcv-8D6 binds to the channel as a tetramer but not to the monomer form.
Fig. 4.
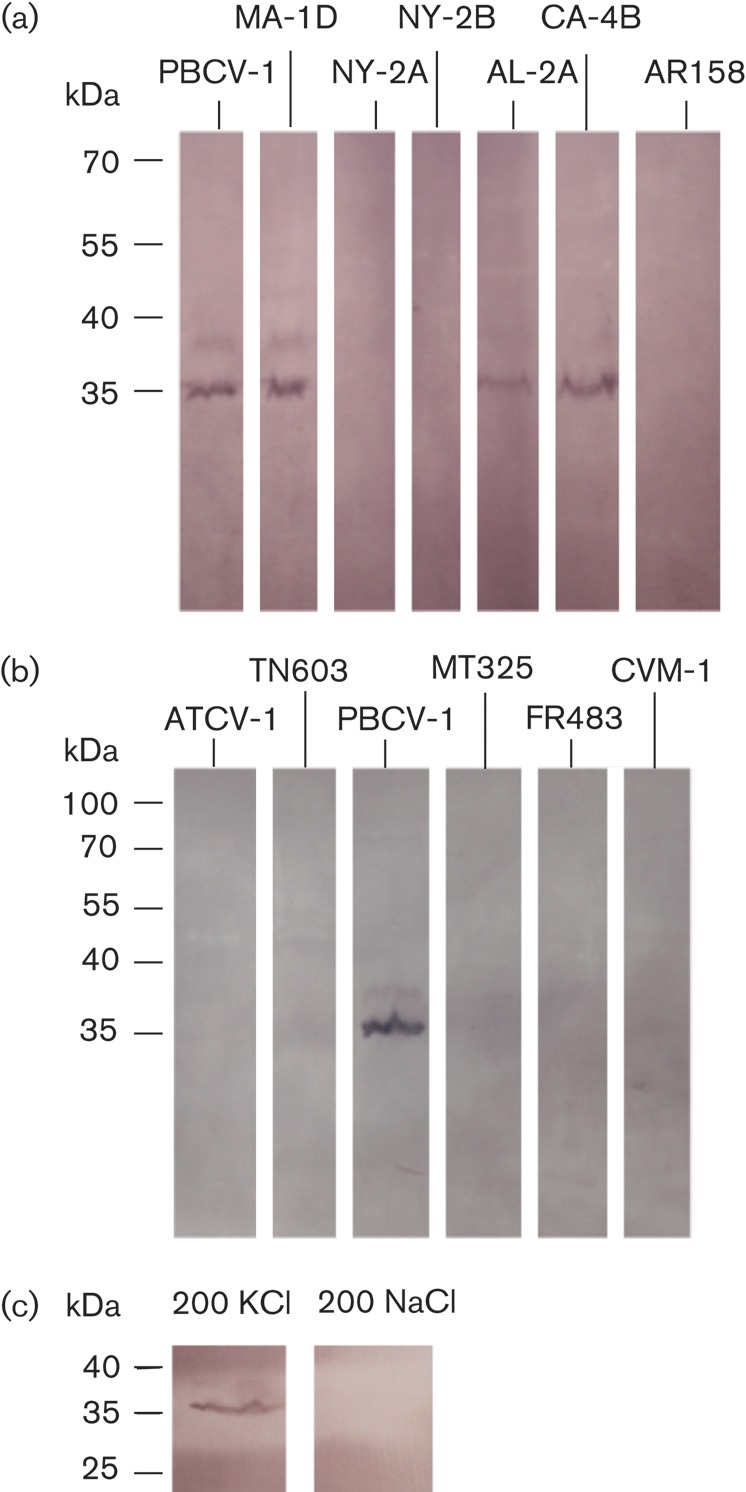
Western blot with protein extracts from different chloroviruses. (a) Viruses that infect C. variabilis. (b) Viruses that infect C. heliozoae (ATCV-1, TN603) and M. conductrix (MT325, FR483 and CVM-1). Virus PBCV-1 was the positive control in (b). (c) Western blot of KcvPBCV-1 protein in the presence of 200 mM KCl or NaCl in SDS buffer. Molecular size markers are shown on the left (kDa).
Specificity of anti-Kcv-8D6 mAb
To determine whether anti-Kcv-8D6 antibody reacted with the Kcv protein in a background of endogenous proteins, we disrupted yeast cells expressing KcvPBCV-1 and separated the proteins by electrophoresis. KcvPBCV-1 is a reference Kcv channel that we routinely express in yeast. Previous experiments have shown that these cells synthesize this channel, which differs by 5 aa from KcvMA-1D (Fig. 2), and express the channel as a functional tetramer in the plasma membrane (Balss et al., 2008). A Western blot (Fig. 3) showed that anti-Kcv-8D6 bound exclusively to a single polypeptide with the expected size of the Kcv tetramer, indicating that anti-Kcv-8D6 recognizes KcvPBCV-1. Furthermore, the antibody was specific. This conclusion was supported by finding that an extract from yeast cells that were transformed with another Kcv channel protein (KcvAR158) did not cross-react with anti-Kcv-8D6 (Fig. 3). KcvAR158 is from a chlorovirus that encodes a truncated Kcv-type channel (Fig. 2; Fitzgerald et al. 2007b); its expression in yeast was not recognized by the antibody (Fig. 3).
Fig. 2.
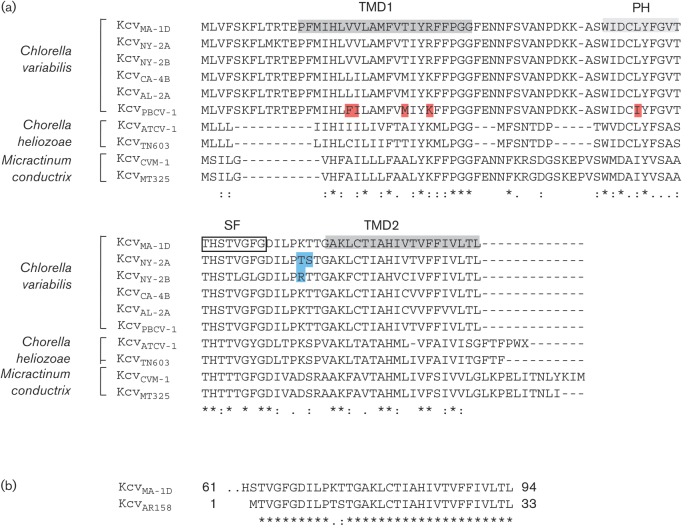
Alignment of viral channels used in this study. (a) Viruses MA-1D, NY-2A, NY-2B, CA-4B, AL-2A and PBCV-1 infect C. variabilis, viruses ATCV-1 and TN603 infect C. heliozoae, and viruses MT325 and CVM-1 infect M. conductrix. The structural domains including the first (TMD1) and second (TMD2) transmembrane domains, the pore helix (PH) and the selectivity filter (SF) are indicated in the KcvMA-1D sequence. The amino acids in KcvNY-2A and KcvNY2B that differ from KcvMA-1D are shaded in blue. The amino acids in KcvPBCV-1 that differ from KcvMA-1D are shaded in red. (b) Alignment of Kcv from virus MA-1D and truncated KcvAR158 from virus AR158; both viruses have the same host, C. variabilis. The NCBI Protein accession numbers of the K+ channel sequences are: KcvMA-1D, AGE54840; KcvNY-2A, YP_001497532; KcvNY-2B, AAQ16142; KcvCA-4B, AAQ16134; KcvAL-2A, AAQ16130; KcvPBCV-1, Q84568; KcvTN603, AGE59882; KcvMT325, ABT13737; KcvCVM1, AEG51 769.
Fig. 3.
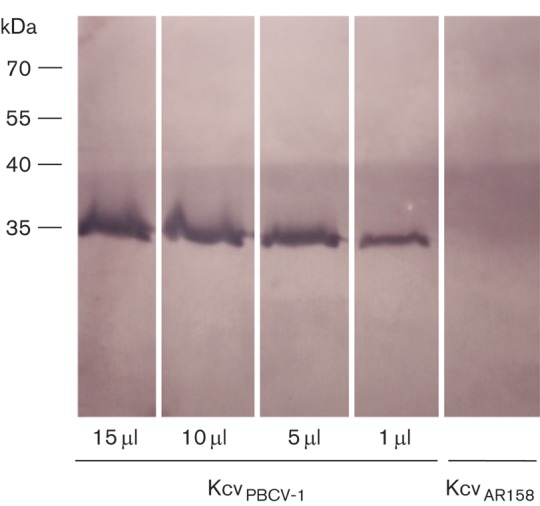
Western blot of protein extracts from S. cerevisiae expressing KcvPBCV-1 (1–15 µl of extract) or expressing KcvAR158, as indicated. Molecular size markers are shown on the left (kDa).
Chloroviruses package Kcv
The positive reaction of anti-Kcv-8D6 for the KcvMA-1D and KcvPBCV-1 channels prompted us to test for Kcv channels in these two purified viruses, as well as from other chloroviruses; all these viruses infect the same host, C. variabilis. The selected virions (Fig. 2) were disrupted in SDS and subjected to SDS-PAGE. Coomassie blue-stained gels revealed at least 50–60 proteins with molecular masses ranging from 10 to ~135 kDa (Skrdla et al., 1984; Dunigan et al., 2012). The polypeptides were blotted onto nylon filters and stained with anti-Kcv-8D6 antibody. The antibody specifically reacted with one prominent band of about 35 kDa in four viruses (PBCV-1, MA-1D, AL-2A and CA-4B; Fig. 4a).
The antibody did not react with proteins from chlorovirus AR158 because AR158 codes for a truncated Kcv protein (Fig. 2) (Fitzgerald et al. 2007a), which is unlikely to form a tetramer (Fig. 2). Surprisingly, Kcv proteins from two NC64A chloroviruses, NY-2A and NY-2B, did not react with anti-Kcv-8D6 (Fig. 4a). This negative result also occurred with a fourfold-higher concentration of virus protein extract (results not shown). Hence, the absence of a reaction with NY-2A and NY-2B was presumably not a matter of protein concentration. Functional assays during virus infection and their sensitivity to inhibitors of Kcv channels indicated that viruses NY-2A and NY-2B contain active Kcvs. However, functional differences exist between Kcvs from PBCV-1 and MA-1D and those from NY-2A and NY-2B. For example, Kcvs from NY-2A and NY-2B are blocked by both Ba2+ and Cs+, whereas Kcvs from PBCV-1 and MA-1D are only blocked by Ba2+ in heterologous cells (Kang et al., 2004). There is no reason to believe that PBCV-1 and MA-1D viruses package Kcv in their virions and that NY-2A and NY-2B do not. A more likely explanation is that anti-Kcv-8D6 fails to cross-react with viruses NY-2A and NY-2B. One reason could be that these two channels are not recognized by the antibody because they have a lower SDS resistance, and hence are in the monomer form, than the other Kcv channels. Another possible explanation is related to subtle structural differences between the channel proteins. Scrutiny of the alignments (Fig. 2) indicated that KcvNY-2A and KcvNY-2B differ from the channels that are recognized by the antibody. One interesting difference exists in the short extracellular stretch of residues connecting the selectivity filter to TMD2 (Fig. 2). As the antibody binds to the extracellular side of the channel (see below), this structural deviation between the channels may alter the antigenic properties of the protein.
Chloroviruses that infect different hosts such as C. heliozoae (SAG viruses) or M. conductrix (Pbi viruses) also encode Kcv-type channels (Fig. 2). These channels are predicted to have a similar, but not identical, architecture as the aforementioned channels, but they differ considerably in their primary amino acid sequence from viruses that infect C. variabilis (Fig. 2). Western blot analysis indicated that anti-Kcv-8D6 did not recognize Kcv channels from chloroviruses that infect C. heliozoae or M. conductrix (Fig. 4b). Finally, the antibody also did not react with proteins from chlorovirus FR483, which infects M. conductrix and is the only one of the 41 sequenced chloroviruses that does not encode a Kcv-type channel (Fitzgerald et al., 2007a).
After finding evidence for the Kcv channel in some viruses, we attempted to localize the protein in the particles. For this purpose, ultrathin sections were prepared from C. variabilis cells 6 h after infection with virus PBCV-1. Under these conditions, mature virus particles are easily visible under an electron microscope in the Chlorella cells. To localize the Kcv channels, the thin sections were reacted with anti-Kcv-8D6 as a primary antibody and 10 nm gold-coupled secondary antibodies. This procedure revealed many gold particles associated with virus capsids (Fig. 5a). At higher magnification, gold labels were seen near the periphery of the virus particles (Fig. 5b). A quantitative analysis of 12 thin sections revealed that 80 % of the gold particles were in a similar position within a radius of 140 nm from the centre of individual virions. A histogram showing the distance of a gold particle from the centre of a virus indicated that most were located between 60 and 80 nm from the virus centre. This coincided roughly with the location of the internal membrane of the PBCV-1 particle (Fig. 5c).
Fig. 5.
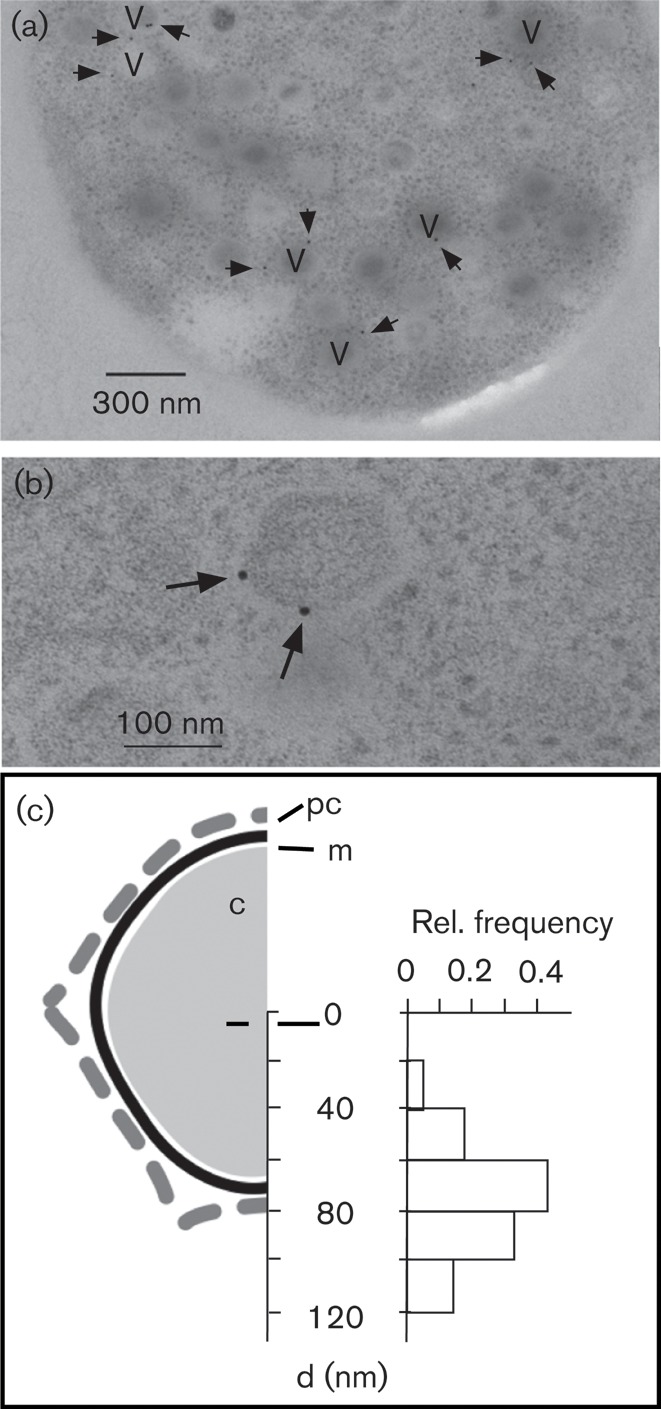
Location of the Kcv channel at the periphery of virus particles. (a) Electron micrograph of a thin section through a C. variabilis cell producing PBCV-1 particles. Virus particles are indicated with a V and gold particles are indicated by arrows. (b) Magnification of an individual virion with two peripheral gold labels. (c) Redrawing (to scale) of one half of a PBCV-1 particle (Zhang et al., 2011) showing the protein coat (pc), membrane (m) and core (c). The histogram shows the relative frequency of the distances (d) where gold particles were detected with respect to the centre of the virus particles.
To further test the specificity of the gold labelling, we also examined thin sections that were treated only with the secondary antibody. Gold particles were occasionally found but only 20 % were within a 100 nm radius of a virus particle. These results confirmed that the Kcv protein is present in virus particles at a low copy number. Their presence at the periphery of the particles is consistent with the idea that the channel is located in the inner membrane of the virus (Yan et al., 2000; Mehmel et al., 2003).
Antibody binds to the extracellular side of the Kcv channel
To determine whether the antibody binds to the extracellular or the cytosolic side of the channel protein, we transiently expressed KcvPBCV-1 in mammalian cells. We have shown previously that expression of the viral K+ channels fused at the C terminus with GFP results in a low number of functional K+ channels appearing in the plasma membrane. The activity of these channels can be detected by patch clamp experiments (Moroni et al., 2002). Fig. 6 shows a confocal image of a COS7 cell expressing KcvPBCV-1–GFP. The reticulate fluorescent pattern in Fig. 6(a) is characteristic of KcvPBCV-1-expressing cells (Balss et al., 2008); under these conditions, the viral protein is most abundant in the secretory pathway including the endoplasmic reticulum. The plasma membrane exhibits no apparent fluorescent signal, even though electrophysiological recordings confirm that a low number of functional channels is indeed present in the plasma membrane (Moroni et al., 2002). When green fluorescent cells, which express the channel, were incubated with anti-Kcv-8D6 as a primary antibody and CF 640R as a secondary antibody, the fluorescence of the red secondary antibody was visible in discrete spots (Fig. 6b). Cells with no green fluorescence and that did not express Kcv produced no red fluorescent signal in the presence of primary and secondary antibodies (not shown). Red fluorescence was also not detected when cells were treated only with the secondary antibody. The low number of fluorescent spots detected in these experiments (in the order of a hundred) was consistent with the number of active Kcv channels routinely estimated by patch clamp in mammalian cells, which express these viral K+ channels. From single-channel conductance studies (Pagliuca et al., 2007) and the mean current recorded in a mammalian cell (e.g. Moroni et al., 2002), we estimated that 50–200 channels were active in the plasma membrane per transfected cell.
Fig. 6.
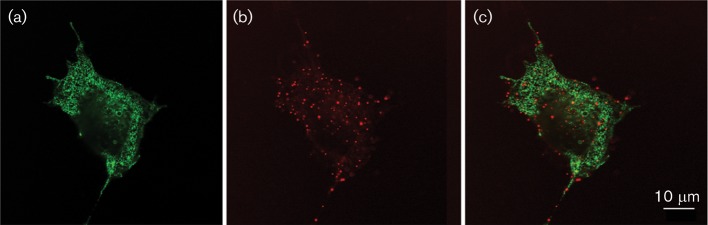
Confocal images of a COS7 cell expressing GFP-tagged KcvPBCV-1. (a) Image of green fluorescence stemming from a Kcv channel in the secretory pathway. (b) Red fluorescence of secondary antibody CF 640R. (c) Overlay of (a) and (b).
The positive reaction of the antibody with the Kcv channel in intact cells suggested that it binds to the extracellular side of the protein. This finding is consistent with the fact that, as mentioned previously, the ‘turret’ domain was the only one with appreciable predicted antigenicity. Also, as the antibody did not recognize the monomeric form of the channel, the antigenic structure presumably results from the display of the four individual turrets in the tetramer.
Conclusion
Several viruses encode and package proteins with ion-channel function in their virions that serve various functions in virus replication (Wang et al., 2011). Although circumstantial evidence supports the view that chloroviruses package a small, virus-encoded K+-channel protein in their virions (Thiel et al. 2010), KcvPBCV-1 was not detected in a PBCV-1 proteome analysis (Dunigan et al., 2012). However, the present experiments establish that KcvPBCV-1 is packaged in the virus particle and that the channel exists as a tetramer, i.e. in its functional form (Pagliuca et al., 2007; Shim et al., 2007). Furthermore, electron microscopy experiments indicated that functional Kcv channels are present in the inner membrane of the viral particle. Collectively, these results support the hypothesis that when the viral membrane fuses with the host plasma membrane during infection, the large conductance of the viral channel short circuits the host membrane, which leads to all subsequent events required for successful infection of the host (Agarkova et al., 2008; Greiner et al., 2009; Thiel et al., 2010). Whilst this hypothesis is compatible with chloroviruses that encode K+ channels, it fails to explain infection by the few chloroviruses that either lack a kcv gene (virus FR483; Fitzgerald et al., 2007a) or have a truncated gene (virus AR158; Fitzgerald et al., 2007b). In the case of virus FR483, the virus has a functional K+ transporter-encoding gene (Greiner et al., 2011). However, expression studies have established that this protein is unlikely to replace the function of the Kcv channel.
Methods
Kcv antibody production.
The hybridoma cell lines producing mAb were obtained commercially (EMBL, Monterotondo, Rome, Italy) from mice immunized with the tetrameric KcvMA-1D protein. The hybridoma cells were grown by standard methods (Harlow & Lane, 1989). Twenty-six different supernatants were tested by Western blot analysis against the tetrameric and the monomeric forms of KcvMA-1D; the monomeric form was obtained by boiling the tetramer for 10 min in 15 % SDS (Pagliuca et al., 2007). mAb clone 8D6 was selected because it recognized the tetrameric form but not the monomeric form of the channel. Purification of 8D6 antibody was performed with protein A according to standard methods (Harlow & Lane, 1989). When tested with other Kcv proteins, the antibody recognized several of them, including PBCV-1 Kcv tetramers.
Cell culture.
COS7 cells were cultivated at 37 °C at ambient 5 % CO2 in DMEM/Ham’s F-12 medium (Dulbecco’s modified Eagle’s medium F-12+10 % FCS+1 % penicillin/streptomycin and l-glutamine). The chlorovirus channel was transiently expressed in cells by transfection with Turbofect (Fermentas/Thermo Fisher Scientific). COS7 cells were therefore grown to 40–60 % confluency on coverslips in 35 mm culture dishes and transiently transfected with 1.0 µg plasmid DNA containing Kcv–GFP according to the manufacturer’s manual. Expression was monitored for more than 24 h after transfection.
Extraction of proteins from viruses.
Viruses were grown and isolated as described previously (Van Etten et al., 1983). An aliquot of virus suspension was centrifuged for 20 min at 13 000 r.p.m. (Biofuge Pico; Heraeus) and the pellet was resuspended in 15 µl 200 mM KCl plus 15 µl IP buffer [8.7 % glycerol, 2.0 % SDS, 62.5 mM Tris/HCl (pH 6.8), 2 % β-mercaptoethanol, 0.01 % bromophenol blue]. The proteins were incubated for 5 min at 95 °C before SDS-PAGE.
Extraction of proteins from yeast.
The KcvPBCV-1 channel was expressed in yeast (SGY1528) as described previously (Balss et al., 2008). One hundred millilitres of a growing yeast suspension (2×108 cells ml−1) was pelleted at 4 °C at 5000 r.p.m. for 20 min (Beckmann model J2-21) and stored at −80 °C. One millilitre of yeast cells was mixed with 100 mg glass beads and vortexed for 10 min. The disrupted cells were gently centrifuged with a manual centrifuge for 2 min and the pellet stored at −20 °C.
Gel electrophoresis.
Protein extracts were separated by electrophoresis according to Laemmli (1970) on a 12.5 % acrylamide gel. A Page Ruler Prestained Protein Ladder (Thermo Fisher Scientific) was used to determine molecular mass.
Western blot analyses of chlorovirus MA-1D protein.
Four microlitres of a KcvMA-1D solution (100 µg ml−1 in lauryldimethylamine oxide) was solubilized with 15 µl buffer comprising 8.7 % glycerol (v/v), 2 % SDS, 62.5 mM Tris/HCl (pH 6.8), 2 % β-mercaptoethanol and 0.01 % bromphenol blue. After 5 min of incubation at 95 °C, the protein samples were separated by SDS-PAGE (12.5 % acrylamide). Separated proteins were transferred to a nitrocellulose membrane and the membrane was submerged overnight with agitation in blocking solution [20 mM Tris/HCl (pH 7.5), 0.9 % (w/v) NaCl, 3 % (w/v) BSA, 0.2 % (v/v) Tween 20] and then washed as follows: 1 min in TBS [20 mM Tris/HCl (pH 7.5), 0.9 % (w/v) NaCl]; 5 min in TBS plus 0.01 % (v/v) Tween 20; 15 min in TBS plus 0.01 % (v/v) Tween 20; and 5 min in TBS. The membrane was incubated in the supernatant collected from hybridoma cell lines producing anti-Kcv 8D6 antibody for 2 h at room temperature, washed as above and incubated with an alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma), diluted 1 : 2000 in TBS plus 3 % (w/v) BSA and 0.01 % (v/v) Tween 20, for 1 h at room temperature. After washing four times as described above, a BCIP/nitro-blue tetrazolium tablet (Bio-Rad) dissolved in H2O was added to the membrane to detect the protein.
Viruses.
Equal amounts of chlorovirus (87.5 µg) were pelleted by centrifugation at 13 000 r.p.m. (Biofuge Pico; Heraeus) for 20 min. The pellets were solubilized in the same buffer that was used for the virus MA-1D protein. After 5 min at 95 °C, the samples were separated by SDS-PAGE (12.5 % acrylamide). Separated proteins were transferred to a PVDF membrane and the membrane was submerged overnight at room temperature in blocking solution [10 mM TBS (pH 9.5) containing 2 % skimmed milk] and then washed four times for 5 min in TBS. The membrane was incubated with anti-Kcv 8D6 antibody (diluted 1 : 1000 in TBS) for 1 h at room temperature, washed as above and incubated with an alkaline phosphatase-conjugated goat anti-mouse IgG (Sigma), diluted 1 : 15 000 in TBS, for 1 h at room temperature. After washing twice for 5 min each in TBS and four times for 10 min each in AP buffer (12.1 g Tris l−1, 5.8 g NaCl l−1, 1 g MgCl2 l−1, pH 9.5), BCIP/nitro-blue tetrazolium was added as above as substrates for colour development.
Light microscopy.
The fluorescence of EGFP and CF 640R (Biotrend) was observed under a confocal microscope (TCS SP 5 II; Leica) with appropriate settings. COS7 cells were grown on coverslips in culture dishes. Cells that had been transiently transfected with KcvPBCV-1–GFP, were washed twice for 5 min with PBS. Subsequently the cells were incubated in 1 ml 5 % BSA in PBS for 30 min. After removing this blocker solution, anti-Kcv-8D6 antibody was added, diluted 1 : 1000 in 1 ml fresh blocker medium, for 1 h. The cells were then washed for 5 min with fresh blocker solution before the secondary antibody, CF 640R, was added, diluted 1 : 500, for 50 min. In the final step, the cells were washed once with fresh blocker medium and twice for 5 min with PBS. The coverslips were removed from the culture dish and placed with PBS on the microscope. GFP was excited with an argon laser at 488 nm and detected at 500–550 nm. The fluorophor CF 640R on the secondary antibody was excited with a helium–neon laser at 633 nm and detected at 650–750 nm.
Electron microscopy.
For high-pressure freezing and freeze substitution for electron microscopy, infected C. variabilis cells were pelleted, loaded on to planchettes (types 241 and 242; Wohlwend) and frozen in a high-pressure freezer (HPM010; Bal-Tec). Subsequent dehydration was performed in a freeze substitution machine (EM AFS2; Leica) using dry acetone supplemented with 0.3 % uranyl acetate (at −85 °C for 16 h) as the substitution medium. After gradually warming to −60 °C over a 5 h period, the samples were washed with dry ethanol for 60 min, infiltrated with Lowicryl HM20 (Polysciences) at −60 °C, embedded and polymerized with UV light inside the EM AFS2 for 48 h (Hillmer et al., 2012). Ultrathin sections were cut on a Leica Ultracut S, post-stained with aqueous uranyl acetate (3 %, w/v)/lead citrate and examined in a JEM1400 (JEOL) transmission electron microscope operating at 80 kV. Micrographs were recorded with a FastScan F214 digital camera (T-VIPS) and contrast/brightness were adjusted with EMMenu 4 (T-VIPS). For immunoelectron microscopy, ultrathin sections were incubated with anti-Kcv-8D6 antiserum (diluted 1 : 50 or 1 : 100), followed by incubation with 10 nm gold-coupled secondary antibodies (BioCell GAR10; British BioCell) at a dilution of 1 : 50 in PBS supplemented with 1 % BSA.
Acknowledgements
We thank Dr Sabrina Gazzarrini and Paolo Zuccolini (University of Milan, Italy) for having tested the effect of the antibody on the Kcv currents in Xenopus oocytes. This research was partially supported by the Deutsche Forschungsgemeinschaft (G. T.), by Cariplo 2009-351 and by PRIN 2010CSJX4F (A. M.), by the Stanley Medical Research Institute 11R-0001 (J. V. E), NSF-EPSCoR grant EPS-1004094 (J. V. E), DOE DE-EE0003142 (J. V. E) and the COBRE program of the National Center for Research Resources grant P20-RR15635 (J. V. E).
References
- Agarkova I., Dunigan D. D., Gurnon J., Greiner T., Barres J., Thiel G., Van Etten J. L. (2008). Chlorovirus-mediated membrane depolarization of Chlorella alters secondary active transport of solutes. J Virol 82, 12181–12190 10.1128/JVI.01687-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balss J., Papatheodorou P., Mehmel M., Baumeister D., Hertel B., Delaroque N., Chatelain F. C., Minor D. L., Jr, Van Etten J. L. & other authors (2008). Transmembrane domain length of viral K+ channels is a signal for mitochondria targeting. Proc Natl Acad Sci U S A 105, 12313–12318 10.1073/pnas.0805709105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunigan D. D., Cerny R. L., Bauman A. T., Roach J. C., Lane L. C., Agarkova I. V., Wulser K., Yanai-Balser G. M., Gurnon J. R. & other authors (2012). Paramecium bursaria chlorella virus 1 proteome reveals novel architectural and regulatory features of a giant virus. J Virol 86, 8821–8834 10.1128/JVI.00907-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer F., Poetsch A. (2006). Protein cleavage strategies for an improved analysis of the membrane proteome. Proteome Sci 4, 2 10.1186/1477-5956-4-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Graves M. V., Li X., Feldblyum T., Hartigan J., Van Etten J. L. (2007a). Sequence and annotation of the 314-kb MT325 and the 321-kb FR483 viruses that infect Chlorella Pbi. Virology 358, 459–471 10.1016/j.virol.2006.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald L. A., Graves M. V., Li X., Feldblyum T., Nierman W. C., Van Etten J. L. (2007b). Sequence and annotation of the 369-kb NY-2A and the 345-kb AR158 viruses that infect Chlorella NC64A. Virology 358, 472–484 10.1016/j.virol.2006.08.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohns F., Käsmann A., Kramer D., Schäfer B., Mehmel M., Kang M., Van Etten J. L., Gazzarrini S., Moroni A., Thiel G. (2006). Potassium ion channels of Chlorella viruses cause rapid depolarization of host cells during infection. J Virol 80, 2437–2444 10.1128/JVI.80.5.2437-2444.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S., Severino M., Lombardi M., Morandi M., DiFrancesco D., Van Etten J. L., Thiel G., Moroni A. (2003). The viral potassium channel Kcv: structural and functional features. FEBS Lett 552, 12–16 10.1016/S0014-5793(03)00777-4 [DOI] [PubMed] [Google Scholar]
- Gazzarrini S., Kang M., Epimashko S., Van Etten J. L., Dainty J., Thiel G., Moroni A. (2006). Chlorella virus MT325 encodes water and potassium channels that interact synergistically. Proc Natl Acad Sci U S A 103, 5355–5360 10.1073/pnas.0600848103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzarrini S., Kang M., Abenavoli A., Romani G., Olivari C., Gaslini D., Ferrara G., van Etten J. L., Kreim M. & other authors (2009). Chlorella virus ATCV-1 encodes a functional potassium channel of 82 amino acids. Biochem J 420, 295–303 10.1042/BJ20090095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner T., Frohns F., Kang M., Van Etten J. L., Käsmann A., Moroni A., Hertel B., Thiel G. (2009). Chlorella viruses prevent multiple infections by depolarizing the host membrane. J Gen Virol 90, 2033–2039 10.1099/vir.0.010629-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiner T., Ramos J., Alvarez M. C., Gurnon J. R., Kang M., Van Etten J. L., Moroni A., Thiel G. (2011). Functional HAK/KUP/KT-like potassium transporter encoded by chlorella viruses. Plant J 68, 977–986 10.1111/j.1365-313X.2011.04748.x [DOI] [PubMed] [Google Scholar]
- Harlow E., Lane D. (1989). Antibodies: a Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory [Google Scholar]
- Hillmer S., Viotti C., Robinson D. G. (2012). An improved procedure for low-temperature embedding of high-pressure frozen and freeze-substituted plant tissues resulting in excellent structural preservation and contrast. J Microsc 247, 43–47 10.1111/j.1365-2818.2011.03595.x [DOI] [PubMed] [Google Scholar]
- Jeanniard A., Dunigan D. D., Gurnon J. R., Agarkova I. V., Kang M., Vitek J., Duncan G., McClung O. W., Larsen M. & other authors (2013). Towards defining the chloroviruses: a genomic journey through a genus of large DNA viruses. BMC Genomics 14, 158 10.1186/1471-2164-14-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M., Moroni A., Gazzarrini S., DiFrancesco D., Thiel G., Severino M., Van Etten J. L. (2004). Small potassium ion channel proteins encoded by chlorella viruses. Proc Natl Acad Sci U S A 101, 5318–5324 10.1073/pnas.0307824100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 10.1038/227680a0 [DOI] [PubMed] [Google Scholar]
- Mehmel M., Rothermel M., Meckel T., Van Etten J. L., Moroni A., Thiel G. (2003). Possible function for virus encoded K+ channel Kcv in the replication of chlorella virus PBCV-1. FEBS Lett 552, 7–11 10.1016/S0014-5793(03)00776-2 [DOI] [PubMed] [Google Scholar]
- Moroni A., Viscomi C., Sangiorgio V., Pagliuca C., Meckel T., Horvath F., Gazzarrini S., Valbuzzi P., Van Etten J. L. & other authors (2002). The short N-terminus is required for functional expression of the virus-encoded miniature K+ channel Kcv. FEBS Lett 530, 65–69 10.1016/S0014-5793(02)03397-5 [DOI] [PubMed] [Google Scholar]
- Neupärtl M., Meyer C., Woll I., Frohns F., Kang M., Van Etten J. L., Kramer D., Hertel B., Moroni A., Thiel G. (2008). Chlorella viruses evoke a rapid release of K+ from host cells during the early phase of infection. Virology 372, 340–348 10.1016/j.virol.2007.10.024 [DOI] [PubMed] [Google Scholar]
- Pagliuca C., Goetze T. A., Wagner R., Thiel G., Moroni A., Parcej D. (2007). Molecular properties of Kcv, a virus encoded K+ channel. Biochemistry 46, 1079–1090 10.1021/bi061530w [DOI] [PubMed] [Google Scholar]
- Plugge B., Gazzarrini S., Nelson M., Cerana R., Van Etten J. L., Derst C., DiFrancesco D., Moroni A., Thiel G. (2000). A potassium channel protein encoded by chlorella virus PBCV-1. Science 287, 1641–1644 10.1126/science.287.5458.1641 [DOI] [PubMed] [Google Scholar]
- Shim J. W., Yang M., Gu L. Q. (2007). In vitro synthesis, tetramerization and single channel characterization of virus-encoded potassium channel Kcv. FEBS Lett 581, 1027–1034 10.1016/j.febslet.2007.02.005 [DOI] [PubMed] [Google Scholar]
- Skrdla M. P., Burbank D. E., Xia Y., Meints R. H., Van Etten J. L. (1984). Structural proteins and lipids in a virus, PBCV-1, which replicates in a Chlorella-like alga. Virology 135, 308–315 10.1016/0042-6822(84)90188-0 [DOI] [PubMed] [Google Scholar]
- Tayefeh S., Kloss T., Thiel G., Hertel B., Moroni A., Kast S. M. (2007). Molecular dynamics simulation of the cytosolic mouth in Kcv-type potassium channels. Biochemistry 46, 4826–4839 10.1021/bi602468r [DOI] [PubMed] [Google Scholar]
- Thiel G., Moroni A., Dunigan D., Van Etten J. L. (2010). Initial events associated with virus PBCV-1 infection of Chlorella NC64A. Prog Bot 71, 169–183 10.1007/978-3-642-02167-1_7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiel G., Baumeister D., Schroeder I., Kast S. M., Van Etten J. L., Moroni A. (2011). Minimal art: or why small viral K+ channels are good tools for understanding basic structure and function relations. Biochim Biophys Acta 1808, 580–588 10.1016/j.bbamem.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Van Etten J. L. (2003). Unusual life style of giant chlorella viruses. Annu Rev Genet 37, 153–195 10.1146/annurev.genet.37.110801.143915 [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Dunigan D. D. (2012). Chloroviruses: not your everyday plant virus. Trends Plant Sci 17, 1–8 10.1016/j.tplants.2011.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Xia Y., Meints R. H. (1983). Growth cycle of a virus, PBCV-1, that infects Chlorella-like algae. Virology 126, 117–125 10.1016/0042-6822(83)90466-X [DOI] [PubMed] [Google Scholar]
- Wang K., Xie S., Sun B. (2011). Viral proteins function as ion channels. Biochim Biophys Acta 1808, 510–515 10.1016/j.bbamem.2010.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T., Onimatsu H., Van Etten J. L. (2006). Chlorella viruses. Adv Virus Res 66, 293–336 10.1016/S0065-3527(06)66006-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan X., Olson N. H., Van Etten J. L., Bergoin M., Rossmann M. G., Baker T. S. (2000). Structure and assembly of large lipid-containing dsDNA viruses. Nat Struct Biol 7, 101–103 10.1038/72360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Xiang Y., Dunigan D. D., Klose T., Chipman P. R., Van Etten J. L., Rossmann M. G. (2011). Three-dimensional structure and function of the Paramecium bursaria chlorella virus capsid. Proc Natl Acad Sci U S A 108, 14837–14842 10.1073/pnas.1107847108 [DOI] [PMC free article] [PubMed] [Google Scholar]