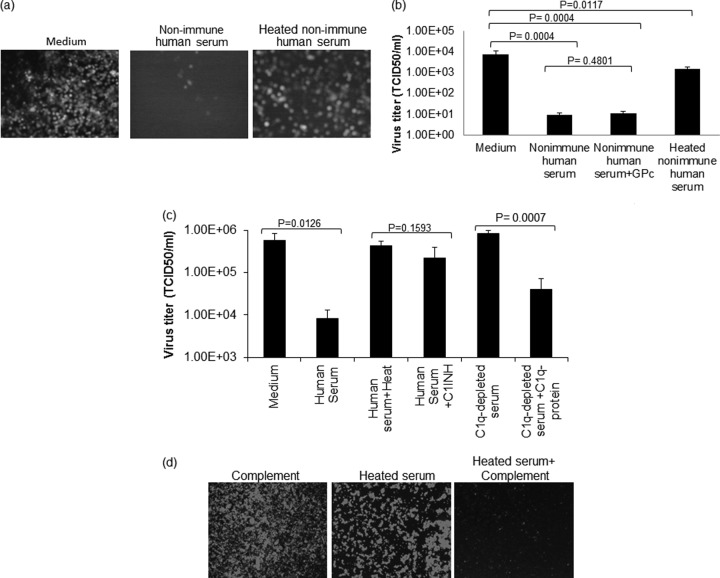
FIG 2.
Neutralization of VSV with nonimmune human serum depends on serum complement. (a and b) VSV-GFP was incubated with nonimmune human serum with or without addition of 10% standard guinea pig complement (GPc), heat-treated (56°C for 30 min) human serum, or medium control. (a) The virus-serum or virus-medium mixture was incubated for 1 h at 37°C. Following incubation, the mixture was overlaid on 12-well plates of Vero cells, and micrographs were taken 24 h postinfection. (b) The virus-serum mixture was diluted 10-fold and titrated on 96-well plates of overnight-plated Vero cells for a TCID50/ml determination. (c) VSV-GFP was incubated for 1 h at 37°C with nonimmune human serum, C1INH-treated nonimmune human serum, Clq-depleted human serum, or Clq-depleted human serum that was constituted with purified Clq protein or, as a control, with medium only. The virus titer was determined as for panel b. (d) VSV-GFP (1 × 104 TCID50) was incubated for 1 h at 37°C with medium containing 10% guinea pig complement, heated nonimmune serum, or heated nonimmune serum reconstituted with 10% guinea pig complement, followed by plating the mixture on monolayers of Vero cells on 96-well plates. Micrographs were taken 24 h postinfection. All virus neutralization assay experiments were conducted in triplicate, and the error bars show standard deviations.