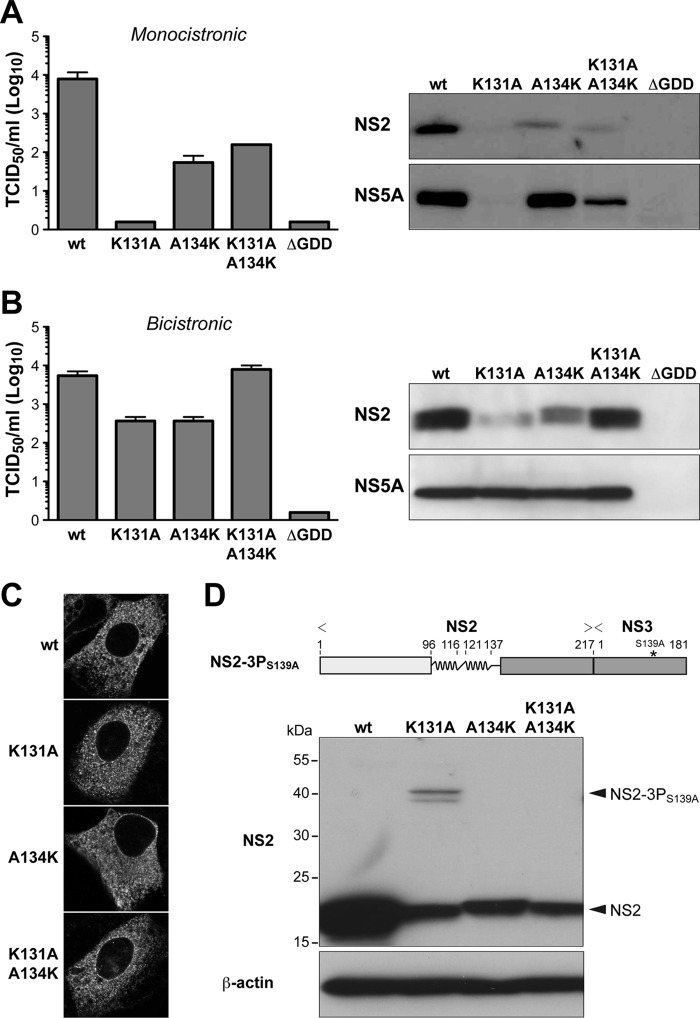
FIG 3.
The positively charged residue in H2 is important for HCV polyprotein processing. (A and B) The indicated mutations were introduced into a monocistronic full-length construct harboring NS2 from HCV genotype 1a isolate H77 (11) (A) or a bicistronic full-length construct harboring an encephalomyocarditis virus (EMCV) internal ribosome entry site (IRES) between NS2 from HCV genotype 1a isolate H77 and NS3 (11) (B). Virus infectivity, quantified shown on the left, was assessed by 50% tissue culture infectious dose (TCID50) determination at 72 h postelectroporation, as described previously (16). Immunoblot analyses using monoclonal antibodies (MAbs) 6H6 against NS2 (8, 11) and 9E10 against NS5A (16) (both kindly provided by Charles M. Rice) are shown on the right. (C) NS2 expressed in the context of the different bicistronic viruses was examined 48 h postelectroporation by immunofluorescence microscopy using MAb 6H6. (D) A CMV promoter-driven construct allowing expression of NS2 and the protease domain of NS3 with an inactivating S139A mutation in its catalytic site (NS2-3PS139A) was derived from HCV genotype 1a isolate H77 and is illustrated on the top. Processing of the wild-type (wt) construct as well as of constructs harboring the indicated mutations in NS2 H2 was analyzed by transient transfection into U-2 OS cells and immunoblotting using MAb 6H6 against NS2. Mouse monoclonal AC-15 against β-actin was used as a loading control. The positions of NS2-3PS139A and of processed NS2 are indicated on the right.