FIG 2.
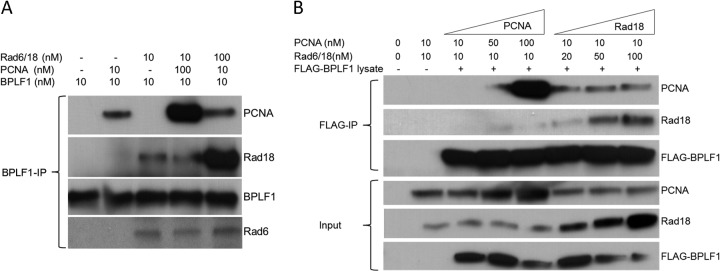
Rad18 interacts with BPLF1 but does not compete with PCNA for a common binding site on BPLF1. (A) BPLF1 interacts with Rad18 in vitro. BPLF1 and Rad6/Rad18 protein complex expressed and purified from E. coli were incubated together at equimolar concentrations (lanes 1 to 3) at room temperature for 1 h and then immunoprecipitated with anti-BPLF1 antibody, and Western blots were probed with Rad18, PCNA, and BPLF1 antibodies. Results showed that BPLF1 interacts with Rad18 independently of PCNA. Additionally, the BPLF1 protein (10 nM) was incubated with either Rad18 (10 nM) and 10-fold excess of PCNA (100 nM) (lane 4) or PCNA (10 nM) and RAD18 (100 nM) (lane 5) and subjected to immunoprecipitation with BPLF1 antibody. (B) PCNA and Rad18 do not compete for a common binding site on BPLF1. A semi-in vivo assay was used. FLAG-BPLF1 was expressed in H1299 cells, and whole-cell lysates were incubated with increasing concentrations of purified PCNA and Rad18 at room temperature for 1 h. Reaction mixtures were immunoprecipitated with FLAG antibody and probed for PCNA, Rad18, and BPLF1. Results indicate no competition between Rad18 and PCNA for BPLF1 binding.
