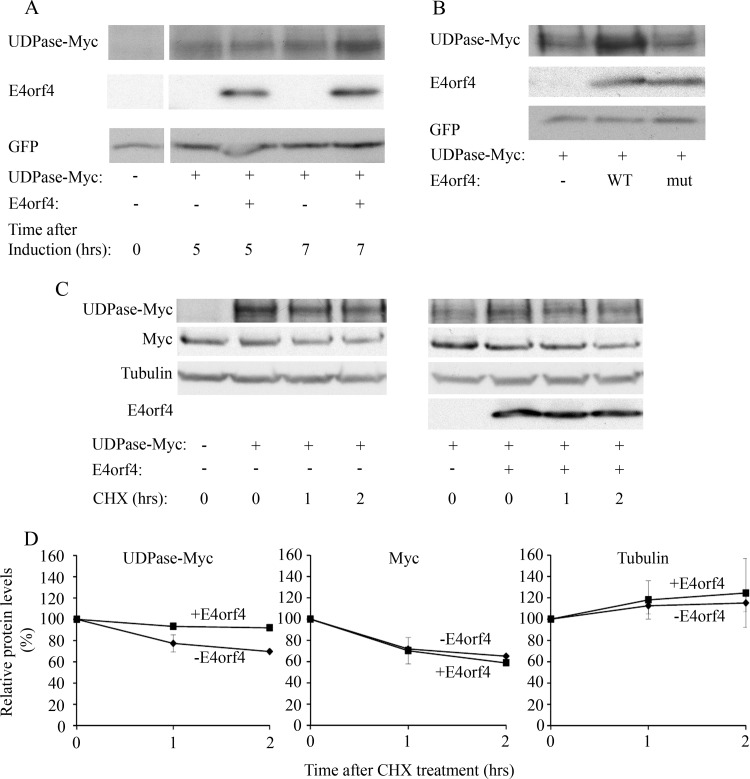
FIG 5.
E4orf4 increases UDPase protein levels. (A) UDPase-Myc was expressed in 293T cells together with inducibly expressed E4orf4 and with GFP. The transfection was carried out in duplicate. One day after transfection, E4orf4 expression was induced by the addition of 5 μg/ml doxycycline to the cells (+E4orf4), and a duplicate plate was treated with ethanol (-E4orf4). The cells were harvested 5 and 7 h later, and equal amounts of proteins were separated by SDS-PAGE. A Western blot was stained with the indicated antibodies. Densitometry of the blot and normalization to the levels of the GFP transfection control revealed that UDPase levels increased by 40% in the presence of E4orf4 after 7 h induction. (B) Plasmids expressing UDPase-Myc and GFP were transfected into 293T cells together with an empty vector or a vector expressing WT E4orf4 or the R81F84A mutant, which does not bind PP2A-B55α (mut). The cells were harvested 1 day after transfection, and protein levels of UDPase-Myc, E4orf4, and GFP were determined by Western blot analysis using specific antibodies. (C) UDPase-Myc was expressed in 293T cells with or without E4orf4. Cycloheximide (CHX; 50 μg/ml) or an equal volume of ethanol was added to the medium 1 day after the transfection, and cells were harvested at 0, 1, and 2 h after addition of the drug. The levels of UDPase-Myc, endogenous Myc, alpha-tubulin, and E4orf4 were visualized by Western blotting. (D) Protein levels were determined by densitometry of the Western blots. Protein levels at time zero were defined as 100%, and relative protein levels are shown in the graphs. Diamonds, samples with empty vector; squares, samples with E4orf4. Error bars represent the standard errors from two independent experiments. Errors smaller than 0.03 are not depicted.