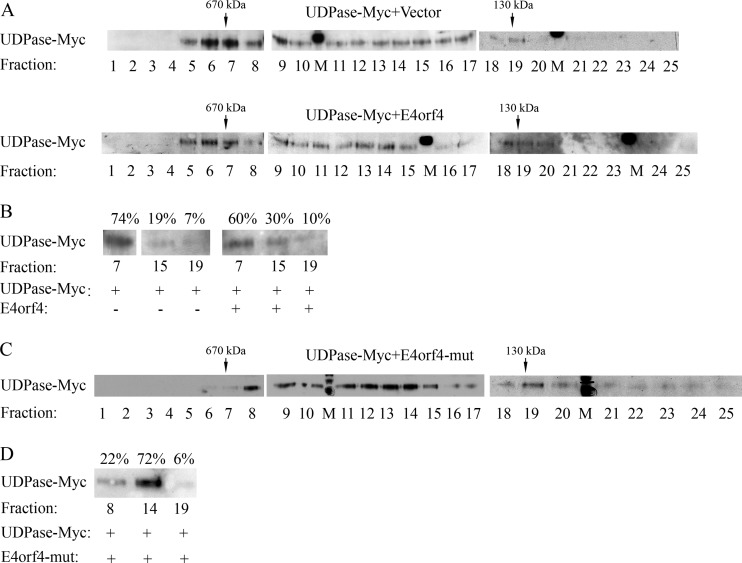
FIG 7.
A high-molecular-weight UDPase-containing complex is partially dissociated in the presence of E4orf4, regardless of the ability of E4orf4 to bind PP2A. (A) Protein extracts from 293T cells expressing UDPase-Myc in the presence or absence of E4orf4 were separated independently on a Superose 6 column. Twenty-five fractions were collected from each run, and 10% of each fraction were loaded onto SDS gels and subjected to Western blot analysis with antibodies to the Myc tag. In addition, thyroglobulin and BSA dimers were chromatographed separately on the column as molecular mass markers (670 and 130 kDa, respectively). M denotes a marker lane. (B) Fractions 7, 15, and 19 from the two column runs whose results are shown in panel A were loaded onto one SDS gel, and a blot was stained with Myc tag-specific antibodies. The intensities of UDPase-Myc protein bands were quantified by densitometry and the sum of UDPase-Myc levels in the 3 fractions of each column run was defined as 100%. The percentage of UDPase-Myc in each fraction is shown above the blot. (C and D) A column run of protein extracts expressing UDPase-Myc in the presence of the R81F84A E4orf4 mutant (mut) was analyzed as described for panels A and B. The results are representative of 3 independent experiments.