FIG 8.
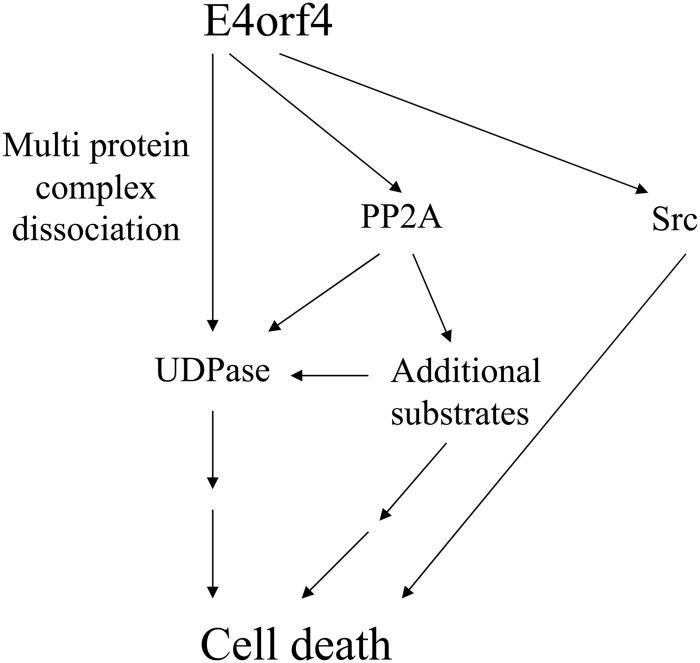
A model of the interaction between UDPase, PP2A-B55α, and E4orf4, and their contribution to induction of cell death by E4orf4. E4orf4 affected UDPase in at least two ways. First, E4orf4 enhanced UDPase levels in a PP2A-dependent manner, and the increased UDPase levels enhanced E4orf4-induced cell death. Since high levels of UDPase alone did not cause cell death, we suggest that other substrates of PP2A were required for enhancing E4orf4 toxicity in parallel with UDPase or in cooperation with it. The finding that UDPase levels were not much influenced by an E4orf4 mutant that bound Src but not PP2A suggests that UDPase cooperated with the E4orf4-PP2A but not the E4orf4-Src pathway. Second, independently of PP2A, E4orf4 dissociated high-molecular-weight complexes that included UDPase. Because the Ynd1 cytoplasmic tail in yeast appears to act as a scaffold that binds E4orf4 and several proteins of the secretory pathway and mediates E4orf4 toxicity (29), we hypothesize that dissociation of a complex tethered to the UDPase cytosolic tail may contribute to induction of cell death. The Src- and UDPase-dependent pathways may provide partially overlapping contributions to E4orf4-induced cell death as they both interact, physically or functionally, with the protein trafficking machinery.