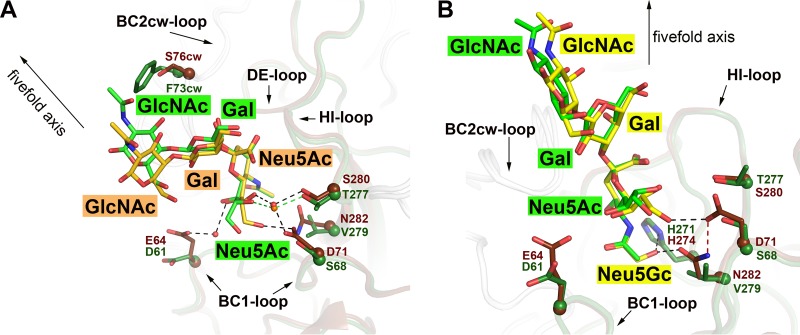
FIG 4.
Comparison of the sialyloligosaccharide binding pockets of HPyV9 and LPyV VP1 proteins. (A) The structure of HPyV9 VP1-3SLN is superimposed onto the structure of LPyV VP1-3SLN (PDB accession number 4MBZ). HPyV9 VP1 and 3SLN are shown as in Fig. 3. For LPyV VP1, one monomer is shown in forest green and other monomers are shown in gray. The side chains are shown in stick representation, and the corresponding C-α atoms are shown as spheres. The 3SLN bound to LPyV VP1 is shown in stick representation; nitrogen and oxygen atoms are colored blue and red, respectively, and carbon atoms are colored forest green. Only residues forming contacts with 3SLN that differ between the two viruses are shown. Water molecules are represented as red and orange spheres for HPyV9 and LPyV, respectively. Hydrogen bonds are shown as black and green dashed lines for HPyV9 and LPyV, respectively. The 5-fold axis of the VP1 pentamer is indicated to depict the orientation of the superimposed pentamers. (B) The structure of HPyV9 VP1-3GSLN is superimposed onto the structure of LPyV VP1-3SLN (PDB accession number 4MBZ). HPyV9 VP1 and 3GSLN are shown as in Fig. 3C. LPyV VP1 and 3SLN are shown as described for panel A. Histidine is present in the binding sites of both viruses (H274 in HPyV9 and H271 in LPyV), but only in HPyV9 does it coordinate the hydroxyl group of Neu5Gc, which also interacts with the side chain of N282 of HPyV9 VP1. Hydrogen bonds are shown as black dashed lines. D71 is present in the proximal vicinity and stabilized the side chain of the N282 via a hydrogen bond (red dashed line).