FIG 5.
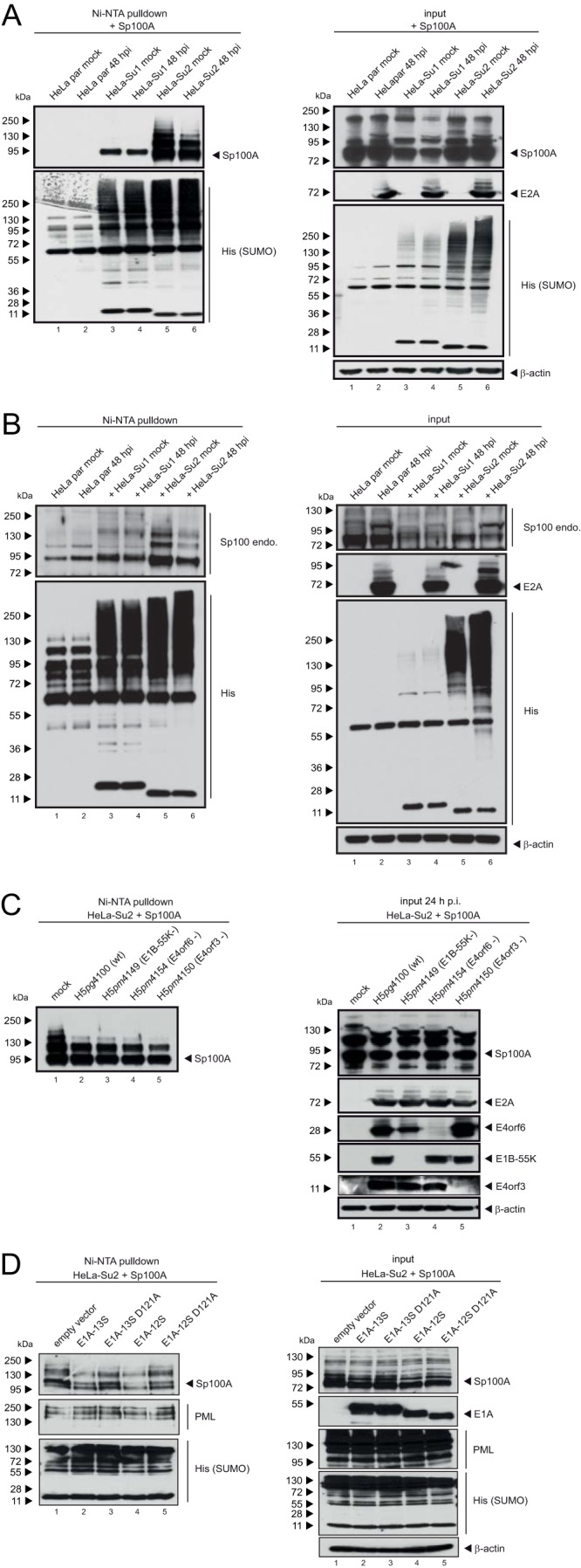
SUMO-2 chains of Sp100 are shortened during Ad infection. Parental HeLa cells and HeLa cells stably expressing 6His-SUMO-1 or 6His-SUMO-2 were transfected with 2 μg of pFlag-Sp100A and then superinfected with wt virus (H5pg4100) (A) or a mutant virus lacking E4orf3 (H5pm4150), E4orf6 (H5pm4154), or E1B-55K (H5pm4149) (C) at a multiplicity of infection of 20 FFU/cell at 8 h posttransfection, as indicated, or cotransfected with 3 μg E1A-13S, E1A-12S, E1A-13S-D121A, or E1A-13S-D121A (D). (B) To monitor endogenous Sp100 (Sp100 endo.), the cells were infected with wt virus (H5pg4100) at a multiplicity of infection of 20 FFU/cell without transfection. For panels A to C, whole-cell lysates were prepared with guanidinium chloride buffer at 48 h posttransfection, subjected to Ni-NTA purification of 6His-SUMO conjugates, and fractionated in a 4 to 12% gradient gel before immunoblot analysis. Input levels of whole-cell lysates and Ni-NTA-purified proteins were detected using pAb GH3 (anti-Sp100), pAb PML NB100-59787, MAb M-58 (anti-E1A), MAb B6 (anti-E2A), MAb 6×His, MAb Flag-M2 (anti-Flag), MAb AC-15 (anti-β-actin), MAb 2A6 (anti-E1B-55K), MAb RSA3 (anti-E4orf6), and MAb 6A11 (anti-E4orf3). Molecular masses in kDa are indicated on the left, and specific proteins are indicated on the right.
