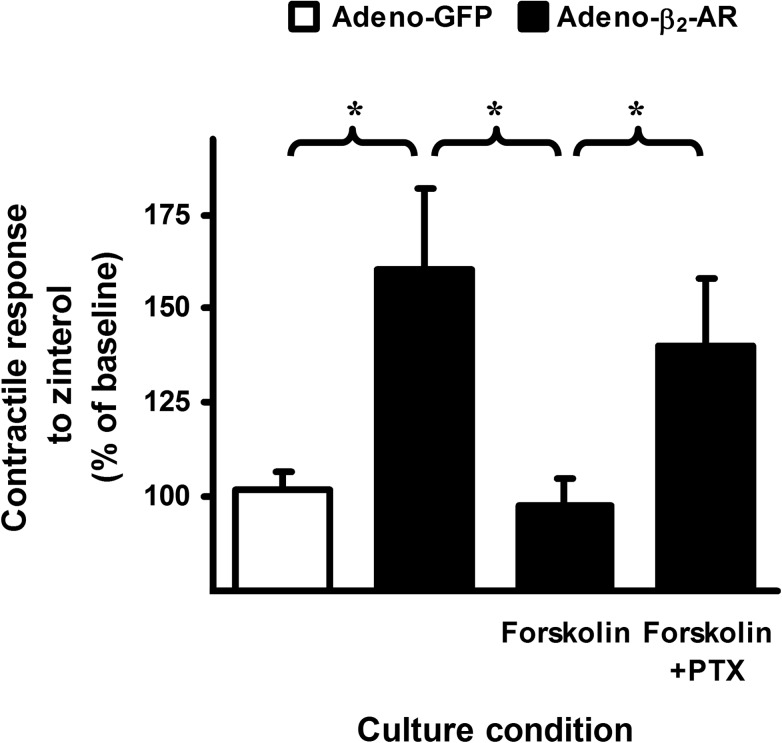
FIGURE 4.
Addition of forskolin reconstitutes functional coupling of β2-AR to Gi protein in cultured β2-AR knock-out mouse cardiomyocytes induced with human β2-AR. Cardiomyocytes from β2-AR knock-out mice were infected with adeno-GFP (white bar) or adeno-β2-AR (black bars) and cultured for 24 h in the presence or absence of forskolin (1 μm) and/or PTX (0.75 μg/ml) as indicated. Cells were transferred to a perfusion chamber, electrically paced, and subjected to stimulation with zinterol (0.2 μm, a concentration without an inotropic effect in freshly isolated cardiomyocytes from WT mice, see Fig. 1A in Ref. 26). Steady-state contractility was measured. Data (mean ± S.E., n = 10–15 cells from 5 to 9 hearts for each data point) are expressed as percentages of the basal contractility. *, p < 0.05. Zinterol (0.2 μm) did not increase contractility in cells infected with adeno-GFP demonstrating no β1-AR stimulatory effect at this concentration. In cells infected with adeno-β2-AR and cultured in the absence of forskolin, the inotropic response produced by zinterol stimulation was the result of a pure β2-AR-Gs-mediated effect because β2-AR and Gi proteins were functionally uncoupled. In cells infected with adeno-β2-AR in the presence of forskolin, the coupling of β2-AR to Gi protein was reestablished. Therefore, the cardiomyocytes were unresponsive to zinterol as if they were freshly isolated WT β2-AR+ cells when β2-AR-Gi coupling was intact. In cells infected with adeno-β2-AR in the presence of forskolin and PTX, the coupling of β2-AR to Gi protein still occurred, but Gi had lost its function and could no longer negatively regulate β2-AR-Gs activation by zinterol.