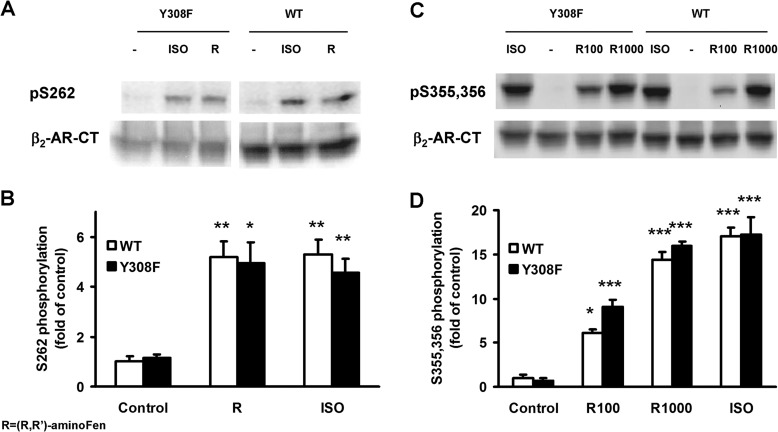
FIGURE 8.
(R,R′)-AminoFen induces phosphorylation of β2-AR and β2-AR Y308F mutant at the GRK and the PKA sites in HEK stable cell lines. Confluent cultures of HEK-β2-AR cells and HEK-β2-AR Y308F cells were incubated in serum-free medium for 3 h and then stimulated with vehicle control (−), ISO (1 μm), or (R,R′)-aminoFen (R, 1 μm; R100 for WT, 0.2 μm; R100 for Y308F, 1 μm; R1000 for WT, 2 μm; R1000 for Y308F, 10 μm) for 5 min at 37 °C. Phosphorylated β2-AR was detected by phosphosite-specific antibodies against Ser(P)-262 for PKA sites and Ser(P)-355,356 for GRK sites. Total β2-AR was detected after stripping and reprobing the membrane with the β2-AR-CT antibody. A, immunoblots of Ser(P)-262-β2-AR and total β2-AR in response to agonist stimulation, and B, averaged data (normalized to total β2-AR). C, immunoblots of Ser(P)-355,356-β2-AR and total β2-AR in response to agonist stimulation, and D, averaged data. Data are expressed as fold increase over control (means ± S.E. in at least three independent experiments). *, p < 0.05; **, p < 0.01; ***, p < 0.001 versus vehicle controls (two-way analysis of variance with post hoc t test). No significant differences were found for all within-group comparisons between WT and Y308F, p > 0.05.